Definition Of Quarantine In Pharmaceutical Industry
Quarantine The status of starting or packaging materials intermediates or bulk or finished products isolated physically or by other effective means while a decision is awaited on. Times Sunday Times 2009 Definition of industry.

Overview Of The Fda S Drug Recall Process
An active ingredient that is used in the fabrication of a drug that is of non-biological origin and that is listed in Schedule C to the Act.

Definition of quarantine in pharmaceutical industry. Quantity of any drug produced during a given cycle of manufactureIf the manufacturing process is continuous the batch originates in adefined period of time during which the manufacturing conditions arestable and have not been modified. All definitions in section 201 of the act shall apply to the regulations in this part. The status of materials isolated physically or by other effective means pending a decision on their subsequent approval or rejection.
Customers definition of quality is the only one. For example flammable goods such as ethanol would be stored in this area. Guidance for Industry.
72 Receipt and Quarantine 73 Sampling and Testing of Incoming Production Materials 74 Storage 75 Re-evaluation 8 Production and In-Process Controls 81 Production Operations 82 Time Limits 83 In-process Sampling and Controls 84 Blending Batches of Intermediates or APIs 85 Contamination Control. This is fine for a company making garden pots but not so good when the products being made are pharmaceuticals and can even cause death. The pharmaceutical industry had provided only limited investment because few applications were considered to be nearly ready for clinical use.
A Quarantine of drug products before release by the quality control unit. Pharmaceutical quality system processes in a visual manner. 1Drug and narcotic control standards 2Drug industry standards.
B Storage of drug products under appropriate conditions of. Quarantine area for storing goods that have not yet been inspected or tested. WHO Library Cataloguing-in-Publication Data Quality assurance of pharmaceuticals.
A Act means the Federal Food Drug and Cosmetic Act as amended secs. Quarantine Lifecycle. In the food drug and medical device industry it is critical that good procedures are in place to ensure a controlled and consistent performance.
An active ingredient that is used in the fabrication of a pharmaceutical. B Complaint means any written electronic or oral communication that alleges deficiencies related to the identity quality durability reliability safety. The documented verification that all aspects of a facility utility or equipment that can affect product quality operated as.
The operation of Quarantine Stations at ports of entry establishment of standards for medical examination of persons destined for the United States and administration of interstate and foreign quarantine regulations which govern the international and interstate movement of persons animals and cargo. Quality Production Laboratory Materials Facilities and Equipment Packaging and Labeling. Materials stored in Quarantine cannot be used or released until approved by QC.
Definitions - Important Terms Inactive ingredient excipient. For the purpose of these guidelines this definition also includes. Office of Communications Division of Drug Information.
A compendium of guidelines and related materials. Q7 Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients. D Management responsibilities within the.
Any substance or mixture of substances intended to be used in themanufacture of a pharmaceutical dosage form and that when used inthe production of a drug becomes an active ingredient of that drugSuch substances are intended to furnish pharmacological activity orother direct effect in the diagnosis cure mitigation treatment orprevention of disease or to affect the structure. 1040 et seq as amended 21 USC. 2 Good manufacturing practices and inspection.
Some warehouses have a Dangerous Goods storage area to ensure the safety of staff and the facility. Additional copies are available from.

Covid 19 How Pharma Can Adapt Blue Matter Consulting

Covid 19 How Pharma Can Adapt Blue Matter Consulting
What The New Coronavirus Means For The Pharmaceutical Industry

Covid 19 How Pharma Can Adapt Blue Matter Consulting
Https Www Who Int Medicines Areas Quality Safety Quality Assurance Expert Committee Isbn9789241209861 Trs986 Pdf

Covid 19 How Pharma Can Adapt Blue Matter Consulting
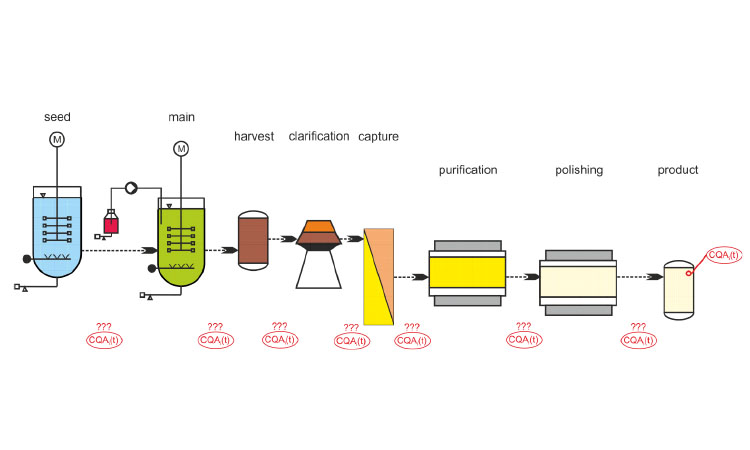
Continuous Manufacturing In Biotech Processes Challenges For Implementation Pharmaceutical Engineering

Inline Spectroscopy Boosts Pharmaceutical Processing And Quality Photonics At Work Apr 2021 Photonics Spectra
Https Apps Who Int Iris Bitstream Handle 10665 340323 9789240020900 Eng Pdf
Https Www Usp Org Sites Default Files Usp Document About Public Policy Apec Good Distribution Practices Pdf
Http Www Who Int Medicines Services Expertcommittees Pharmprep 20160302 Qasterminologydb Pdf
Https Www Who Int Docs Default Source Medicines Norms And Standards Guidelines Mqa Terminology Sept 2020 Pdf Sfvrsn 48461cfc 5

Good Manufacturing Practices Guide For Drug Products Gui 0001 Canada Ca
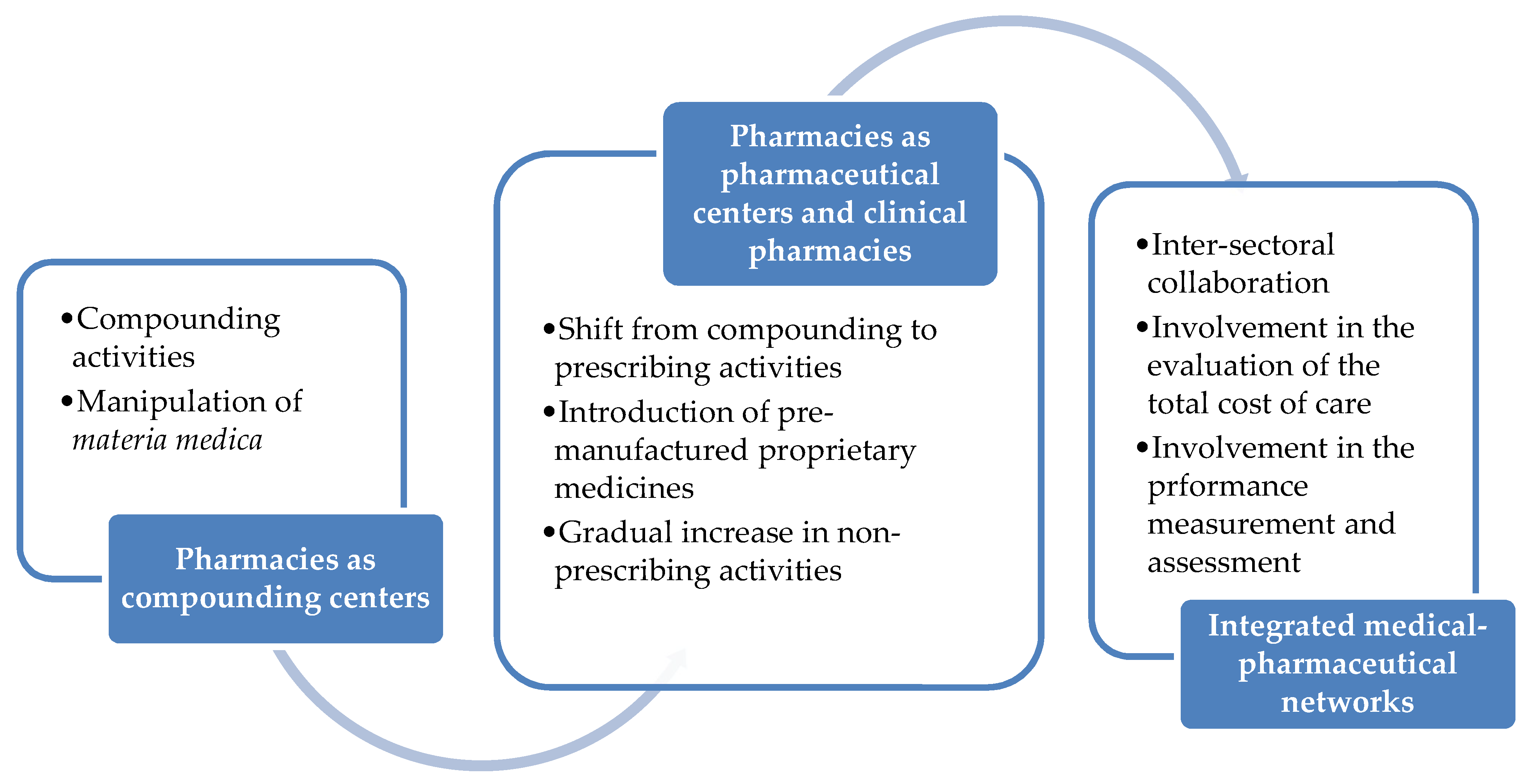
Pharmacy Free Full Text The Role Of Hospital And Community Pharmacists In The Management Of Covid 19 Towards An Expanded Definition Of The Roles Responsibilities And Duties Of The Pharmacist Html

Covid 19 How Pharma Can Adapt Blue Matter Consulting

Overview Of The Fda S Drug Recall Process
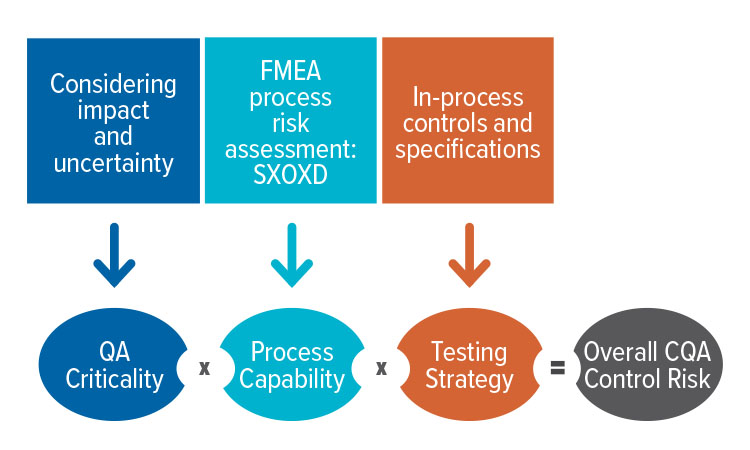
Continuous Manufacturing In Biotech Processes Challenges For Implementation Pharmaceutical Engineering


Post a Comment for "Definition Of Quarantine In Pharmaceutical Industry"