Definition Of Atom With Example
A neutral atom has an equal number of protons and electrons so that the positive and negative charges exactly balance. An atom is defined as the smallest unit that retains the properties of an element.

Matter Atoms Elements Molecules And Compounds Anchor Posters Atoms And Molecules For Kids Molecules Science Poster
A typical atom consists of a nucleus of positively-charged protons and electrically neutral neutrons with negatively-charged electrons orbiting this nucleus.

Definition of atom with example. Bohr Model of Atom. An atom is a particle of matter that uniquely defines achemical element. The nucleus is positively charged and contains one or more relatively heavy particles known as protons and neutrons.
Bit I dont believe theres an atom of meaning in it Lewis Carroll Alices Adventures in Wonderland. What is Atom definition with example. Atom Definition Chemistry The smallest particle of an element which may or may not have an independent existence but always takes place in a chemical reaction is called an atom.
The atom is the basic building block for all matter in the universe. Each electron is negatively charged. The smallest particle of an element that can exist alone or in combination carbon atoms.
An atom consists of a central nucleus that is usually surrounded by one or more electrons. What is Bohr Model. Any of the indivisible particles postulated by philosophers as the basic component of all matter.
For example He and Ne etc. Defining atoms molecules and compounds. Its actually made of two hydrogen atoms and one oxygen atom.
Atoms are extremely small and are made up of a few even smaller particles. Any element listed on the periodic table consists of specific atoms. A tiny particle.
O The Bohr model was an improved form of earlier cubic model 1902 the plum-pudding model 1904 the Saturnian model 1904 and the Rutherford model. However an atom can consist of a single proton ie the protium isotope of hydrogen as. An atom is the defining structure of an element which cannot be broken by any chemical means.
Atom Examples. The key to a molecule is that two or more atoms are bonded together. The smallest unit of an element consisting of at least one proton and for all elements except hydrogen one or more neutrons in a dense central nucleus surrounded by one or more shells of electrons.
The single most important characteristic of an atom is its atomic number usually denoted by the letter Z which is defined as the number of units of positive charge protons in the nucleus. For example the atom isnt an elementary particle. The smallest unit of an element consisting of at least one proton and for all elements except hydrogen one or more neutrons in a dense central nucleus surrounded by one or more shells of electrons.
Atoms fit together with other atoms to make up matter. Hydrogen helium oxygen and uranium are examples of types of atoms. You can also have molecules of a single atom bonded together like two oxygen atoms.
For example water is a molecule made of hydrogen and oxygen. Atom is the smallest particle of an element which can take part in a chemical reaction. Have atoms which have independent existence while atoms of hydrogen nitrogen and oxygen do not exist independently.
An atom is the defining structure of an element which cannot be broken by any chemical means. Definition Diagram Example Published by Admin on August 16 2021 August 16 2021. For example if an atom has a Z of 6 it is carbon while a Z of 92 corresponds to uranium.
A typical atom consists of a nucleus of positively-charged protons and electrically neutral neutrons with negatively-charged electrons orbiting this nucleus. An elementary particle is a particle that is the smallest and most basic component of matter and cannot be broken down into smaller particles. The basic particles that make up an atom are electrons protons and neutrons.
An atom is composed of atoms and these cannot be made or destroyed.

Difference Between Atomic Structure And Crystal Structure Comparison Summary Atomic Structure Crystal Structure Crystals

Atom Infographic Infographic Poster Graphic Design Infographic Atomic Theory

Dispersion Force Teaching Chemistry Force Definition Physical Chemistry

What Is An Ion Chemistry Lessons Definitions Good Tutorials

This Is A Low Prep Atomic Theory Project For Your Middle Or High School Students That Gives Them The Atomic Theory Timeline Project Science Teaching Resources

Complex Compounds Definition Examples Perfect Imperfect Complex Definition Teaching Chemistry Compound Complex Chemistry Classroom

Chemistry Word Wall Word Wall Chemical Changes Words

Difference Between Organic And Inorganic Compounds Definition Structure Properties Chemistry Lessons Chemistry Basics Study Chemistry

Coordinate Covalent Bond Definition Examples Formation And Properties Covalent Bonding Coordinates Chemistry Basics

What Is An Atomic Number Definition And Examples In 2021 Atomic Number Number Definition Mass Number

Delocalized Electron Definition And Examples Chemistry Lessons Chemistry Lesson

Atom Ape Man Atomic Mnemonic For Protons Neutrons Electrons Chemistry Classroom Gcse Chemistry Physical Science Interactive Notebook

What Is An Atom Http Sibeda Com What Is An Atom Protons Neutrons Atom Model Project

Definition And Examples Of A Molecule Covalent Bonding Atoms And Molecules For Kids Molecules

What Is A Molecule Definition And Examples Chemical Bond Molecules Hydrogen Bond
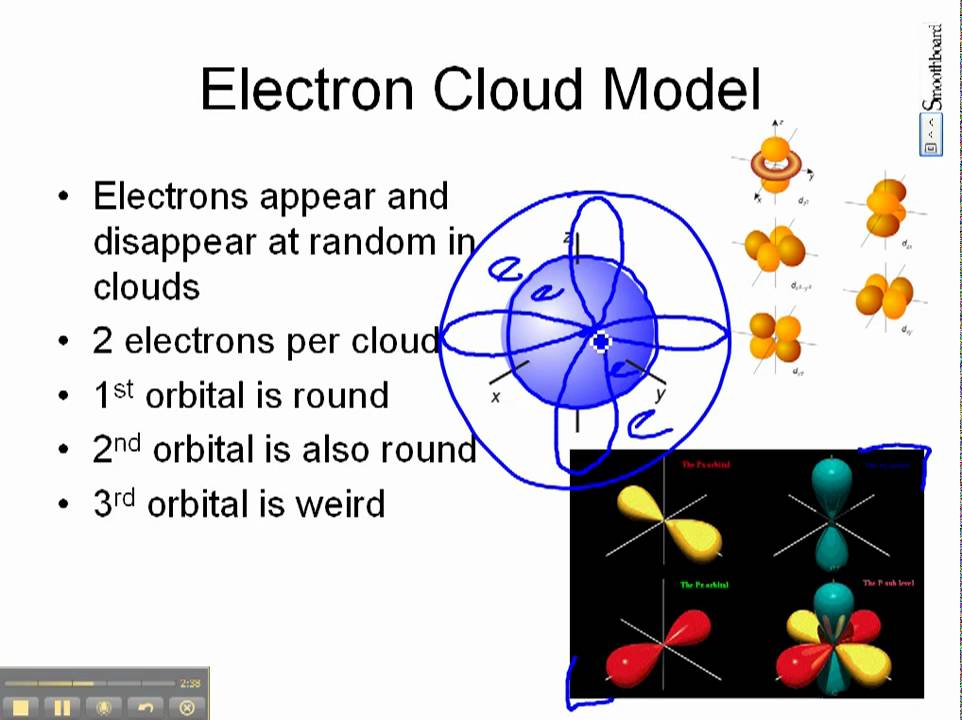
Electron Cloud Model Electrons Elementary Science Clouds

Coordinate Covalent Bond Definition Examples Formation And Properties Covalent Bonding Bond Chemistry

Electronegativity Definition Periodic Trends Examples Importance Electronegativity Difference Digital Kemistry In 2021 Effective Nuclear Charge Definitions Chemistry

Atom Song Teaching Chemistry Atom Song Middle School Science
Post a Comment for "Definition Of Atom With Example"