Definition Of Cell Potential
Biological electricity has the same chemical underpinnings as the current between electrochemical cells and thus can be duplicated outside the body. A cell is a device that.

Cell Potential Easy Science Cell Easy Science Flashcards
The measurement of half-cell potential is used to evaluate.

Definition of cell potential. To optimize cell performance we want to minimize the irreversibilities at a specified current density. The cell potential of an electrochemical cell is the potential difference occurring between the two electrodes of the cell and arises due to the transfer of electrons through the external circuit of a cell that has not reached equilibrium. The difference in potential is localized on the surface membrane.
Definition An action potential is the result of a very rapid rise and fall in voltage across a cellular membrane with every action potential impulse similar in size. In an electrochemical cell the overall potential is the total potential calculated from the potentials of two half cells. The reversible cell potential is the maximum potential that an ideal galvanic device can attain.
A cells standard state potential is the potential of the cell under standard state conditions which is approximated with concentrations of 1 mole per liter 1 M and pressures of 1 atmosphere at 25oC. Every cell of the body has its own membrane potential but only excitable cells - nerves and muscles - are capable to change it and generate an action potential. The cell potential is the sum of electrode potential of the oxidizing and reducing halves.
Biological electricity has the same chemical underpinnings as the current between electrochemical cells and thus can be duplicated outside the body. Because of irreversibilities the potential difference of a practical galvanic device is always lower. The cell potential E cell is the difference in reduction potential between the two half-cells in an electrochemical cell.
The difference in potential energy between the anode and cathode is known as the cell potential in a voltaic cell. Every cell has a cell potential. The half-cell potential measurement can only indicate the corrosion probability at a given location and time but long-term.
Cells actually prefer to be more negative inside. Electrochemical Analysis of Phenols through Charging of Phenol Solutions. Electrode potential Definition.
The formula for cell potential is. The electric potential that arises between the anode and the cathode is due to the difference in the individual potentials of each electrode which are dipped in their respective electrolytes. A potential difference between the contents of a cell or fiber and the extracellular fluid.
The property of potential E is the energy associated with the separationtransfer of charge. The cell potential of an electrochemical cell can be measured with the help of a voltmeter. Potential vulnerability of element surface area to corrosion.
However the individual potential of a half-cell cannot be accurately measured alone. To calculate the standard cell potential for a reaction. Pressure is greater than the legitimate solution pressure of the metal has positive charge and positive electric potential at the electrode of the half-cell.
A resting membrane potential is the difference between the electric potential in the intracellular and extracellular matrices of the cell when it isnt excited. The difference between the concentration of molecules and charge inside and outside a cell is known as membrane potential. This is related to the Gibbs free energy change for the cell reaction given by.
Development of membraneless sodium perborate fuel cell for media flexible power generation. In this case the action of reduction on the electrode occurs. It is negative under resting conditions and becomes positive during an action potential.
The membranes inner surface is charged electronegatively with respect to the outer surface. The response of a nerve or muscle cell to an action potential can vary according to how frequently and for what duration the action potentials are fired. In electrochemistry the potentials of cells and half-cells are thermodynamic quantities that reflect the driving force or the spontaneity of their redox processes.
Every cell has a cell potential. The potential inside a cell membrane measured relative to the fluid just outside. Cell Potential Formula The driving force of the electron flow from anode to cathode shows a potential drop in the energy of the electrons moving into the wire.
Resting potential - the potential difference between the two sides of the membrane of a nerve cell when the cell is not conducting an impulse electric potential potential difference potential drop voltage potential - the difference in electrical charge between two points in a circuit expressed in volts. 121 ΔG n E cell F.

Basic Electrical Terms Includes Electric Current Resistance Voltage Or Potential Difference Circuit Cell Battery Electricity Electrical Engineering Basic

Voltaic Cell Easy Science Chemical Energy Electrochemistry Electrochemical Cell

Transformation Transduction Conjugation Mcat Map Map Screenshot

Best Chemistry Blogs Making Chemistry Easy Tips And Tricks Standard Hydrogen Electrode Definition Constructi Electron Configuration Ideal Gas Law Chemistry

Pin By Microbiology Note On Microbiology Note Medium Definition Distillation Preparation

Galvanic Cell Or Voltaic Cell Construction And Working Electrochemistry Galvanic Cell Electrochemistry Chemistry

1 Electroencephalography Definition Eeg Is A Surface Recording Of The Electrical Activity Of Nerve Cells Of Brain Activities Symmetry Activities Nerve Cell

Emf Of A Cell Nihumathulla Homeopathy Quotes Homeopathic Medicine Homeopathy

Glial Cell Definition Medical Dictionary Definitions Of Popular Terms Defined On Medicinenet Medical Dictionary Cell Definition Medical Definition

Anode Easy Science Easy Science Flashcards Electrochemistry

Hydrogen Bonding Definition Examples And Types Digital Kemistry Hydrogen Bond Bond Molecules

Cytokinesis Definition And Process In Animal And Plant Cells Plant Cell Plant And Animal Cells Cell Organelles

Electrolysis Of Molten Nacl Oxidation Half And Reduction Half Cell Reactions In Electrolytic Cell Oxidation Molten Chemistry
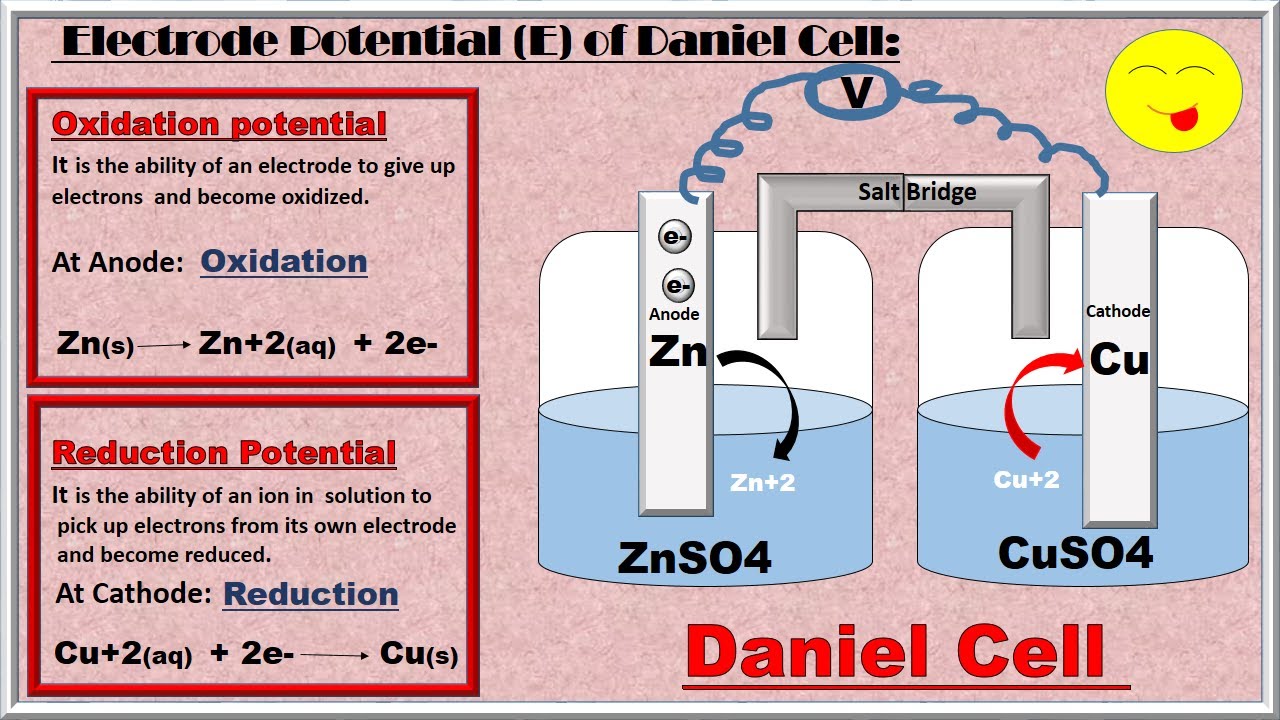
Oxidation Potential And Reduction Potential Chemistry Lessons Reduction Potential Chemistry

Electroplating Easy Science Electroplating Easy Science Electrochemistry

Electrolytic Cell Electrolysis Of Nacl Ap Chemistry Cell Definition Chemistry

Cell Membrane Cell Membrane Membrane Cell

Galvanic Cell And Electrolytic Cell Electrochemical Cells Electrochemical Cell Galvanic Cell Chemistry Lessons

Post a Comment for "Definition Of Cell Potential"