Definition Of Thomson Atom
What is Thomson Model of the Atom Plum Pudding Model Definition 2019-05-22 by Nick Connor The original model of an atom based on the discovery of the electron was proposed by J. Thomson presumed that an electron is two thousand times lighter as compared to proton and thought that an atom is made up of thousands of electrons.
Thomson Atomic Model And Limitations Development Of Atomic Model
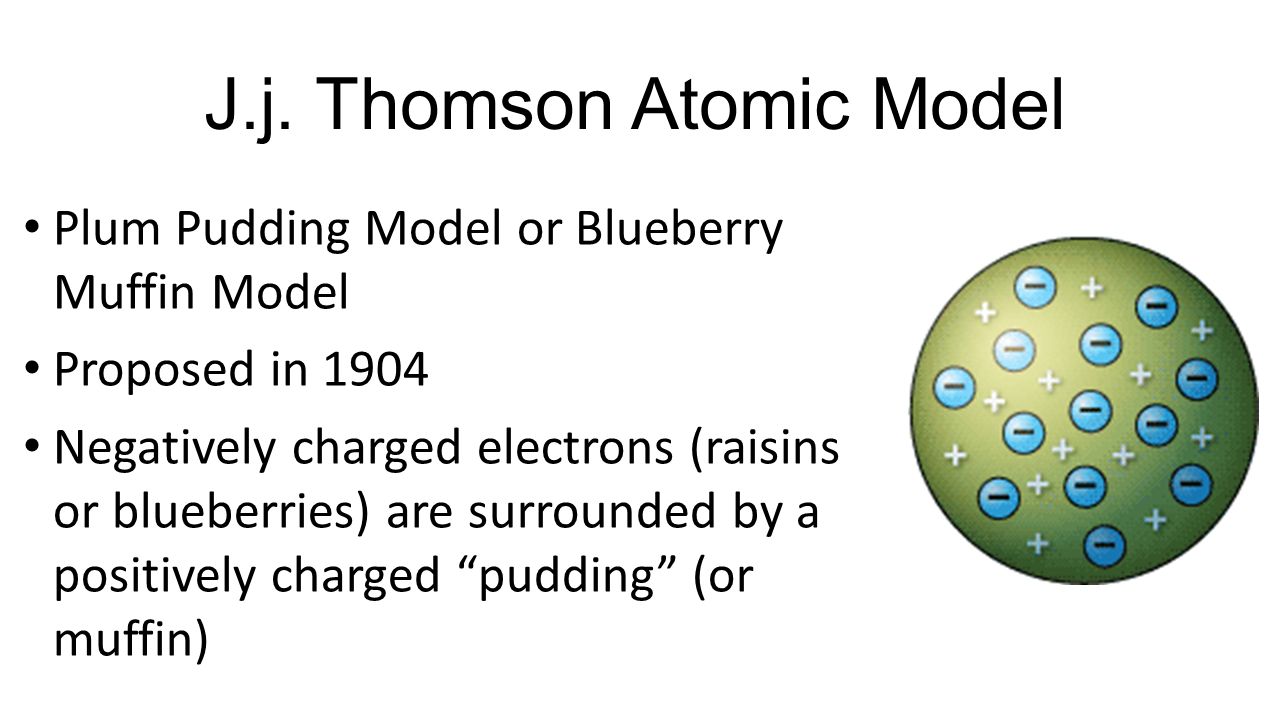
So the atom is electrically neutral Thomsons atomic model was called the plum pudding model.
Definition of thomson atom. The positive and negative charge is equal in magnitude and therefore an atom has no charge as a whole and is electrically neutral. British physicist who discovered the electron in 1897. Thomson in 1904 and is known as the Plum pudding model or the Thomson model of the atom.
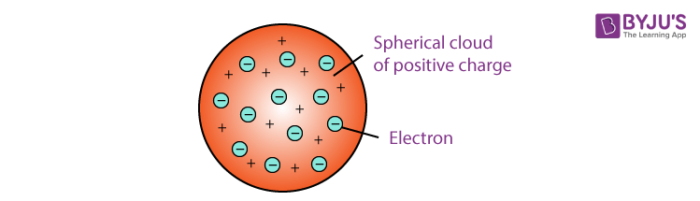
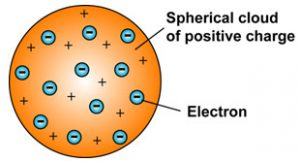
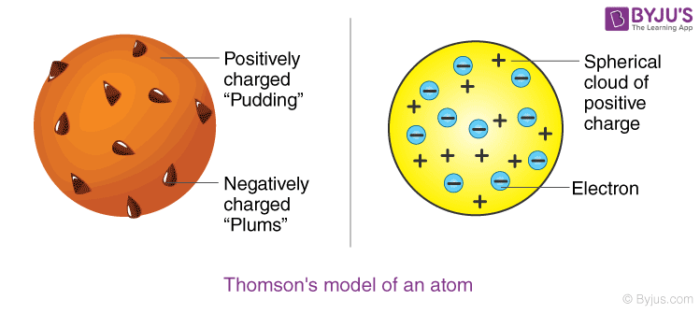
This explains that this atom is a spherical structure made out of a positively charged solid material and the electrons are embedded in that solid. This new nuclear model was an evolution of Dalton s atomic model. This model is also known as the plum pudding model due to its resemblance to a plum pudding.
Definition of Thomsons hypothesis. In this atomic structure model he considered atoms surrounded by a cloud having positive as well as negative charges. While experimenting with cathode rays he deduced that the particles he observed were smaller than an atom.
An atom consists of a large number of electrons held together by a mass with a positive charge equal to. Thomson assumed that an electron is two thousand times lighter than a proton and believed that an atom is made up of thousands of electrons. Sir Joseph John Thomson was a British physicist and Nobel Laureate in Physics.
The Thomson model is a model of the atom proposed in 1904 by Joseph John Thomson. Though several alternative models were advanced in the 1900s by Kelvin and others Thomson held that atoms are uniform spheres of positively charged matter in which electrons are embedded. The existence of protons was also known as was the fact that atoms were neutral in charge.
A largely forgotten legacy of this countrys conquest of the atom. Thomson model of atom is one of the earliest models to describe the structure of atoms. According to Thomson an atom consists of a positive sphere in which negatively charged electrons are embedded in it The negative and positive charges are equal in magnitude.
The smallest particle of an element that can exist either alone or in combination an atom of hydrogen. The atom considered as a source of vast potential constructive or destructive energy. Thomson who discovered the electron in 1897 proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model.
A theory in physics. Thomson atomic model of an atom is reffered to as a plum pudding. In this atomic structure model he stated that the atoms are fenced by a cloud having positive as well as negative charges.
John Dalton 1766-1844 was an English chemist physicist and meteorologist. Thomson also made noteworthy studies of. According to the postulates of Thomsons atomic model an atom resembles a sphere of positive charge with electrons negatively charged particles present inside the sphere.
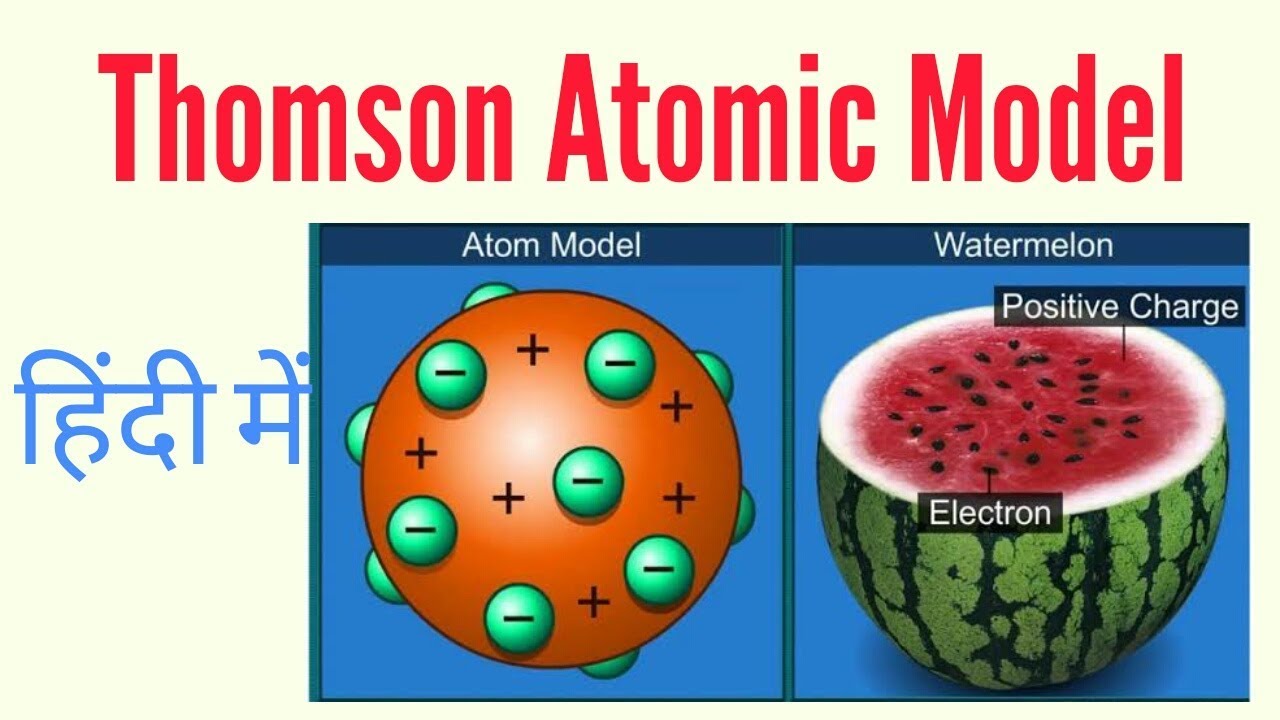
It tells us about different atomic particles. He discovered the electron before discovering the atomic nucleus the first subatomic particle of the atomic structure. Thomsons atomic model resembles a spherical plum pudding as well as a watermelon.
Since the intact atom had no net charge and the electron and proton had opposite charges the next step after the discovery of subatomic particles was to figure out how these particles were arranged in the atom. According to the current theoretical model of the atom it is a dense nucleus covered by a probabilistic cloud of electrons. How was Rutherfords model different from his mentor Thomsons.
Rutherfords model had a small dense positive nucleus. Thomson atomic model earliest theoretical description of the inner structure of atoms proposed about 1900 by William Thomson Lord Kelvin and strongly supported by Sir Joseph John Thomson who had discovered 1897 the electron a negatively charged part of every atom. In 30-140 characters give your own definition of an atom.
The electron was discovered by JJ. The smallest piece of an element that maintains the identity of that element is known as an atom. Atom is made up of positive charge and electrons are embedded like plum pudding or watermelon model.
JJ Thomson Model It is a subatomic model of an atom. Thomsons model of the atom had a diffuse positive charge so the particles should not have been deflected very far. Thomson proposed that the atom is composed of electrons surrounded by a soup of positive charge to balance the electrons negative charges.

Thomson Atomic Model Description Image Britannica

Jj Thomson S Plum Pudding Model Negatively Charged Particles Are Surrounded By A Positively Charged Ball Thomson Atomic Model Thomson Atom Plum Pudding Model

What Is The Plum Pudding Atomic Model Universe Today
J J Thomson Model Of An Atom Class 9 Structure Of An Atom

Explain The Thomson S Model Of An Atom With A Neat Diagram Brainly In
Thomson Atomic Model In Hindi Youtube

Plum Pudding Model Of The Atom What Is It Who Discovered It Electrical4u

Atomic Models Thomson S Atomic Model And Rutherford S Atomic Model

Chemistry Class 9th Chapter 4 Structure Of The Atom Module Thomson S Atomic Model Youtube
Thomson Atomic Model Plum Pudding Model Postulates Limitations
J J Thomson Atomic Model Ppt Video Online Download
Learn Thomson S Model Of Atom In 2 Minutes
Learn Thomson S Model Of Atom In 2 Minutes
J J Thomson The History Of The Atom

The Plum Pudding Model How A Flawed Idea Was Instrumental In Our Understanding Of The Atom

Plum Pudding Model Of The Atom What Is It Who Discovered It Electrical4u

Difference Between Thomson And Rutherford Model Of Atom Comparison Summary Study Chemistry Chemistry Study Guide Rutherford Model



Post a Comment for "Definition Of Thomson Atom"