Definition Of Atom Bbc Bitesize
What is the Structure of an Atom. In ancient Greece most people thought that matter was made up of combinations of four elements.
An atom with an overall charge due to the loss of gain of electrons.

Definition of atom bbc bitesize. Developing the atom Early ideas of matter. An atom is the smallest piece of an element that can exist. An atom is the smallest part of an element that can exist.
Atoms are extremely small and are made up of a few even smaller particles. Atoms are very small. Updated January 24 2020.
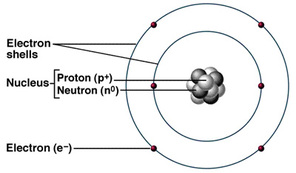
7 million atoms joined together in a straight line would be about 1mm long. An ion is an atom that has lost or gained one or more electrons. This is surrounded by electrons arranged in shells.
Electronegativity is the property of an atom which increases with its tendency to attract the electrons of a bond. Remember this definition as. Everything is made of atoms.
The nucleus contains protons and neutrons. Atoms of each element are represented by their own chemical symbol. Visit the BBC bitesize link.
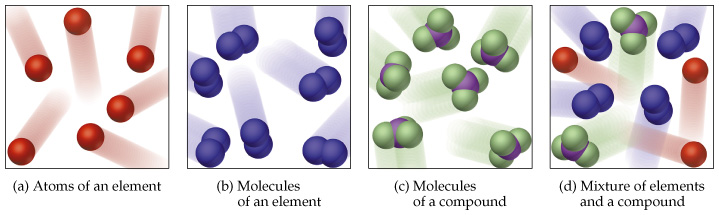
Learn about the differences between atoms elements and compounds with BBC Bitesize KS3 Science. BBC Bitesize Structure of the atom. A mixture contains different substances that are not chemically joined to each other.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Scheme of work Combined Science. Earth air fire and water.
A mixture of iron filings and sulfur powder can easily be separated using a magnet. Movement respiration sensitivity growth reproduction excretion and nutrition. The Ancient Greek theory has been credited to several different scholars but is most often attributed to Democritus 460370 BC and his mentor Leucippus.
BBC Bitesize Practical Skills Question Answer What is the definition of accurate. BBC Bitesize Covalent bonding and the periodic table. BBC Bitesize Handling radioactive materials.
Atoms contain protons neutrons and electrons. The nucleus is tiny compared to the atom as a whole. If two bonded atoms have the same electronegativity values as each other they share electrons equally in a covalent bond.
An impure substance made from different elements or compounds mixed together that are not chemically joined. The atom is the basic building block for all matter in the universe. Results which are accurate are close to the true value.
The electrons are arranged in shells around the nucleus. For example a packet of sweets may contain a mixture of different coloured sweets. An atom has a central nucleus.
This handy mnemonic is a way for children to remember the seven processes that supposedly define life. Scheme of work Chemistry - Bonding structure and the properties of matter 732k BBC Bitesize Ionic compounds and the periodic table. The basic particles that make up an atom are electrons protons and neutrons.
Chemistry Single Science Atomic structure and the periodic table. The radius of an atom is about 01 nm 1 10-10 m the. Synergy Explaining change 98k.
What is the definition of reliable sometimes called. Usually the electrons in a chemical bond are more attracted to one atom the more electronegative one than to. Structure of the atom - Atomic structure and properties relating to bonding - National 4 Chemistry Revision - BBC Bitesize.
Mass number and atomic number are two important pieces of information about an atom. The word atom actually comes from Ancient Greek and roughly translates as indivisible. BBC Bitesize Acidic alkaline or neutral.
The model described the atom as a tiny dense positively charged core called a nucleus around which the light negative constituents called electrons circulate at some distance. The smallest component of an element having the chemical properties of the element consisting of a nucleus containing combinations of neutrons and protons and one or more electrons bound to the nucleus by electrical attraction. What is an Atom.
All atoms have electrons. Structure of the atom - Atomic structure and properties relating to bonding - National 4 Chemistry Revision - BBC Bitesize 2021 Your Bibliography. The number of protons determines the identity of the element.
Atoms fit together with other atoms to make up matter. All atoms have a nucleus the big bit in the middle.

Gcse Science Revision Chemistry The Nuclear Model Youtube

Bbc Bitesize Ks3 Chemistry Atomic Structure Youtube

Bbc Bitesize Ks3 Chemistry Introduction To Atoms And Elements Youtube

Structure Of A Sodium Atom Gcse Chemistry Science Revision Chemistry Periodic Table

Bbc Gcse Bitesize Evidence For Atomic Structure Atomic Structure Atom Model Science Revision

Mass Number Easy Science Mass Number Number Definition Science Student

Learn About The Structure Of Atoms And How Elements Are Arranged In The Periodic Table With Bbc Bitesize Gcse Biology Diagrams Gcse Chemistry Atomic Structure
Yr 8 Topic 5 Materials Amazing World Of Science With Mr Green

Rutherford S Planetary Model Rutherford Model Atomic Theory Atom

Table Showing Symbols And Molecular Models Of Three Hydrogen Isotopes Gcse Chemistry Teaching Chemistry Chemistry

Table Showing The Diagram Electron Configuration And Periodic Table Group Of Fluorine Neon Sodium And Calciu Electron Configuration Chemistry Gcse Chemistry
Https Www Ncpontefract Ac Uk Wp Content Uploads 2017 04 Btec Applied Science Ila New Pdf
Yr 8 Topic 5 Materials Amazing World Of Science With Mr Green

Definition And Examples Of A Molecule Covalent Bonding Atoms And Molecules For Kids Molecules

C1 1 Atoms Secondary Science 4 All

This Is The Plum Pudding Model It Was Developed By Jj Thompson He Proposed This Because Of The Cat Plum Pudding Model Interesting Science Facts Atomic Theory

Gcse Science Revision Physics Atomic Structure Youtube


Post a Comment for "Definition Of Atom Bbc Bitesize"