Definition Of Quantized Atom
The dynamics of any quantum system are described by a quantum Hamiltonian H. Features of quantum mechanical model - definition.

Relationship Between A Particle And A Field 1 2 Quantum World New Quantum Theoretical Physics
In the above equation as the frequency of radiation increases its energy increases by the increment h.

Definition of quantized atom. To limit the possible values of a magnitude or quantity to a discrete set of values by quantum mechanical rules. Thinking about electrons as probabilistic matter waves using the de Broglie wavelength the Schrödinger equation and the Heisenberg uncertainty principle. Atom smallest unit into which matter can be divided without the release of electrically charged particles.
The existence of quantised electronic energy states is a direct result of the wave-like property of electrons. Many apparently continuous phenomena turn out to be quantized at a very fine level or very small scale. The value of n ranges from 1 to the shell containing the outermost electron of that atom.
Atomic model of electron configurations. Light is quantized into packets of energy. The various quantum numbers characterizing a state of the electron in an atom are.
The set of numbers used to describe the position and energy of the electron in an atom are called quantum numbers. To apply quantum mechanics or the quantum theory to. Quantum numbers are used to define the trajectory and movement of an electron within an atom.
As such the atom is the basic building block of chemistry. Development of Quantum Theory. Plancks quantum idea became the basis for the modern understanding of atomic structure.
Electron spin and the Stern-Gerlach experiment. Each frequency of light in the spectrum corresponds to a particular energy of light and therefore to a particular energy loss by a hydrogen atom since this light energy is quantized. This was the first indication that energy is sometimes quantized on a small scale and earned him the Nobel Prize in Physics in 1918.
In chemistry and atomic physics an electron shell or principal energy level may be thought of as the orbit of one or more electrons around an atom s nucleus. An Introduction to Quantum Numbers. Notably this is a crucial topic in your syllabus.
To subdivide something such as energy into small but measurable increments. N the principle quantm number taking values 123 4etc. The quantum mechanical model describes an orbital as a three-dimensional space around the nucleus within an atom where the probability of finding an electron is the highest.
Apply quantum theory to especially form into quanta in particular restrict the number of possible values of a quantity or states of a system so that certain variables can assume only certain discrete magnitudes. In quantum mechanics angular momentum is quantized. In practical use it refers to the minimum amount of energy required for a change or the minimum value of any physical property in an interaction.
Quantize definition is - to subdivide something such as energy into small but measurable increments. LOrbital quantum number takes the values 012. The hydrogen atom was stable because the possible energy states.
For example the energy of electromagnetic radiation such as light at a given. This text is adapted from Openstax Chemistry 2e Section 63. The values of the conserved quantities of a.
The first quantum number describes the electron shell or energy level of an atom. In other words energy was not continuous it was quantized only certain energies are allowed. The distinct orbitals of electrons in an atom are also a case of quantized energy.
The exact position and the exact velocity of an electron in an atom cannot be determined simultaneously Heisenberg uncertainty principle. Using the quantization of oscillators Planck was able to correctly describe the experimentally known shape of the blackbody spectrum. The energy of electrons in an atom is quantised ie electrons can only have certain specific values of energy.
The wave motion of the electron in this field is described with the help of a differential equation known. Introduction to the quantum mechanical model of the atom. We can combine the observation of the hydrogen atom spectrum with our deduction that light energy is quantized into packets to reach an important conclusion.
Kwŏn tīz To limit a variable or variables describing a physical system to discrete distinct values. Quantized quantizing quantizes Physics. Quantum is the singular form of the word.
Quanta is the plural form of the term. There are four quantum numbers namely principal azimuthal magnetic and spin quantum numbers. It should obey the Schrodinger equation.
It also is the smallest unit of matter that has the characteristic properties of a chemical element. The energy spectrum of a system with such discrete energy levels is said to be quantized. Additionally the quantum numbers of every electron in an atom are combined.
N-1 m sub l orbital magnetic quantum number takes the values -l - l-1 -. The quantum mechanical model of the atom describes the electron as a three-dimensional wave in the electronic field of the positively charged nucleus.
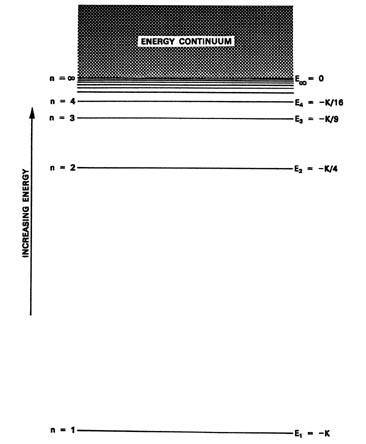
The Hydrogen Atom The Quantization Of Energy

8 2 Quantization Of The Energy Of Electrons Chem 1114 Introduction To Chemistry

Bohr S Theory Of The Hydrogen Atom Physics

3 2 The Quantization Of Energy Chemistry Libretexts

What Is Quantization Of Electric Charge Static Electricity Fundamentals Physics Concepts Youtube

How To Pronounce Quantized Meaning Youtube

What Is Quantization And Why Youtube
Planck S Constant Readings Planck S Constant Photoelectric Effect Wave Particle Duality Bohr Atom Accelerating Electron Produces Em Radiation Light Loses Energy And Spirals Into Nucleus I E Atom Should Not Work The Uv Catastrophe And The Dilemma Of

Important Questions For Class 12 Physics Chapter 12 Atoms Class 12 Important Questions Learn Cbse Https Linear Momentum Chemistry Education Ionization Energy
Illustrated Glossary Of Organic Chemistry Quantized

The Wave Nature Of Matter Causes Quantization Physics

8 2 Quantization Of The Energy Of Electrons Chem 1114 Introduction To Chemistry
Chapter 3 Energy In Bound System Is Quantized

Uum That Can Be Deformed Or Vibrated And Actuators That Cause This Deformation Or Vibration Next The Activity Of These Actuato Quantum Mechanics Quantum Basic

Quantized Field An Overview Sciencedirect Topics
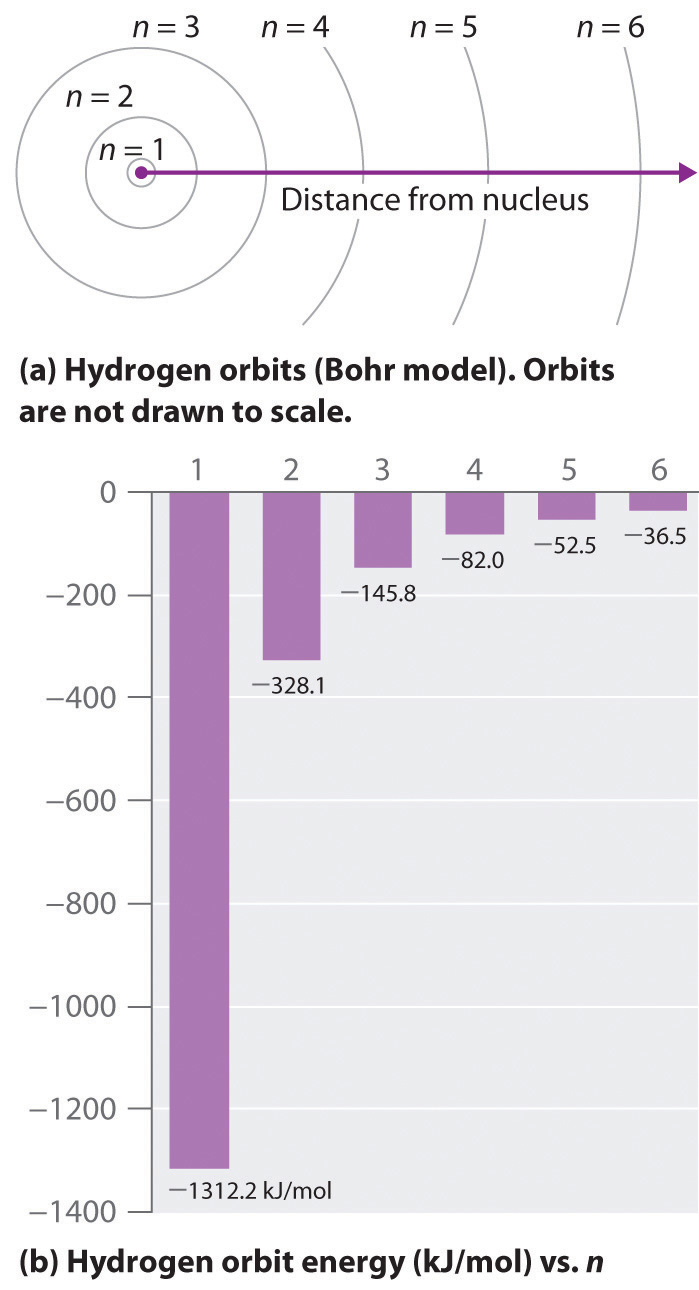
5 4 The Bohr Model Of The Atom Quantized Energy Chemistry Libretexts
Post a Comment for "Definition Of Quantized Atom"