Definition Of Electron Density In Organic Chemistry
Electrophiles are electron poor therefore are electron-loving compounds. It is also named the Gillespie-Nyholm theory after its two main developers Ronald Gillespie and Ronald Nyholm.
Illustrated Glossary Of Organic Chemistry Term
In 2017 a Special Issue named The Molecular Electron Density Theory.
Definition of electron density in organic chemistry. Can be thought of as a two-dimensional slice through a moleculeselectron cloud. Identifying reactive sites in organic molecules amounts to identifying areas of low or high electron density. In x-ray crystallography an interpretation of the diffraction patternas a plot of electron densityversus position in space.
Thus in the process of dehydrogenation the carbon atom undergoes an overall loss of electron density - and loss of electrons is oxidation. Densityin one portion of a moleculedue to electron-withdrawingor electron-donating groupselsewhere in the molecule. Brønsted and Lowry have given us a simple definition of acids and bases.
Now a new Special Issue named The Molecular Electron Density Theory in Organic Chemistry is presented hoping to attract the interest of a. Remember that 95 of organic chemistry can be understood by the following scheme. This is because organic molecules strive for equality for all where equality means that all atoms have an equal share of electrons.
From Quantum mechanics we know that the square of the wave function Ψ at a particular point indicates the probability of finding an electron at that point. The Pauling scale is the most commonly used. The electron density is concentrated in the vicinity of the nucleus showing peaks at the electron density maxima atomic positions.
Often plotted as a topographical map where each atoms electron densityis a mountain peak. One very important key to understanding just about any reaction mechanism is the concept of electron density and how it is connected to the electron movement bond-breaking and bond-forming that occurs in a reaction. The hydroxide ion specifically the electronegative oxygen atom in the hydroxide ion has high electron density due to the polarity of the hydrogen-oxygen bond.
ENC 25 ENCl 30 ΔEN 30 - 25 05. However due to the uncertainty principle its not possible to identify the exact location of an electron at any instant in time. Illustrated Glossary of Organic Chemistry.
410 və-SEP-ər is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. Illustrated Glossary of Organic Chemistry. Distribution of electron density beyond a fixed place such as a single atom lone pair or covalent bond via resonance or inductive effects.
Electron density is a representation of the probability of finding an electron in a specific location around an atom or molecule. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The instantaneous formation of a dipole in the molecule of an organic compound due to the complete transfer of shared pi electron pairs to one of the atoms under the influence of an attacking reagent.
The electron density function ρ r measured in e Å 3 allows us to know the molecular structure from which a crystal is made. An acid is a proton donor and a base is a proton acceptorConsequently electrophiles and nucleophiles are species in organic chemistry that interact very much like acids and bases. In the case of carboncarbon double or triple bonds alkenes and alkynes the region is an area of high electron density.
In general the electron is more likely to be found in regions with high electron density. A Modern View of Molecular was presented in Molecules. The high electron density area will be reactive towards electron-poor deficient molecules.
Illustrated Glossary of Organic Chemistry Electron delocalization delocalization. If you are interested in electronegativity in an organic chemistry context you will find a link at the bottom of this page. Today more than sixty publised manuscripts support the suitability of MEDT.
The Unequal Distribution of Electron Density UDED in Organic Chemistry The following summarizes the UDED concept. An atom or groupwith higher electron density means some aspect of molecular structuresuch as resonanceor inductive effects is shifting negative charge towards this spot in the molecule. Conversely when a carbon atom in an organic compound gains a bond to hydrogen and loses a bond to a heteroatom or to another carbon atom we say that the compound has been hydrogenated or reduced.
Valence shell electron pair repulsion theory or VSEPR theory ˈ v ɛ s p ər v ə ˈ s ɛ p ər VESP-ər. The electron density is the probability or more specifically the probability density of finding an electron in a particular region of space. Molecules react when UDED exists.
A symbol which indicates that an atom or region with a deficiency of electron density often because of resonancedelocalization electronegativitydifferences or inductive effects. Nucleophiles are electron rich compounds also known. Illustrated Glossary of Organic Chemistry.

Ib Chemistry Higher Level Notes Hybridisation

What Is Electron Density Quora
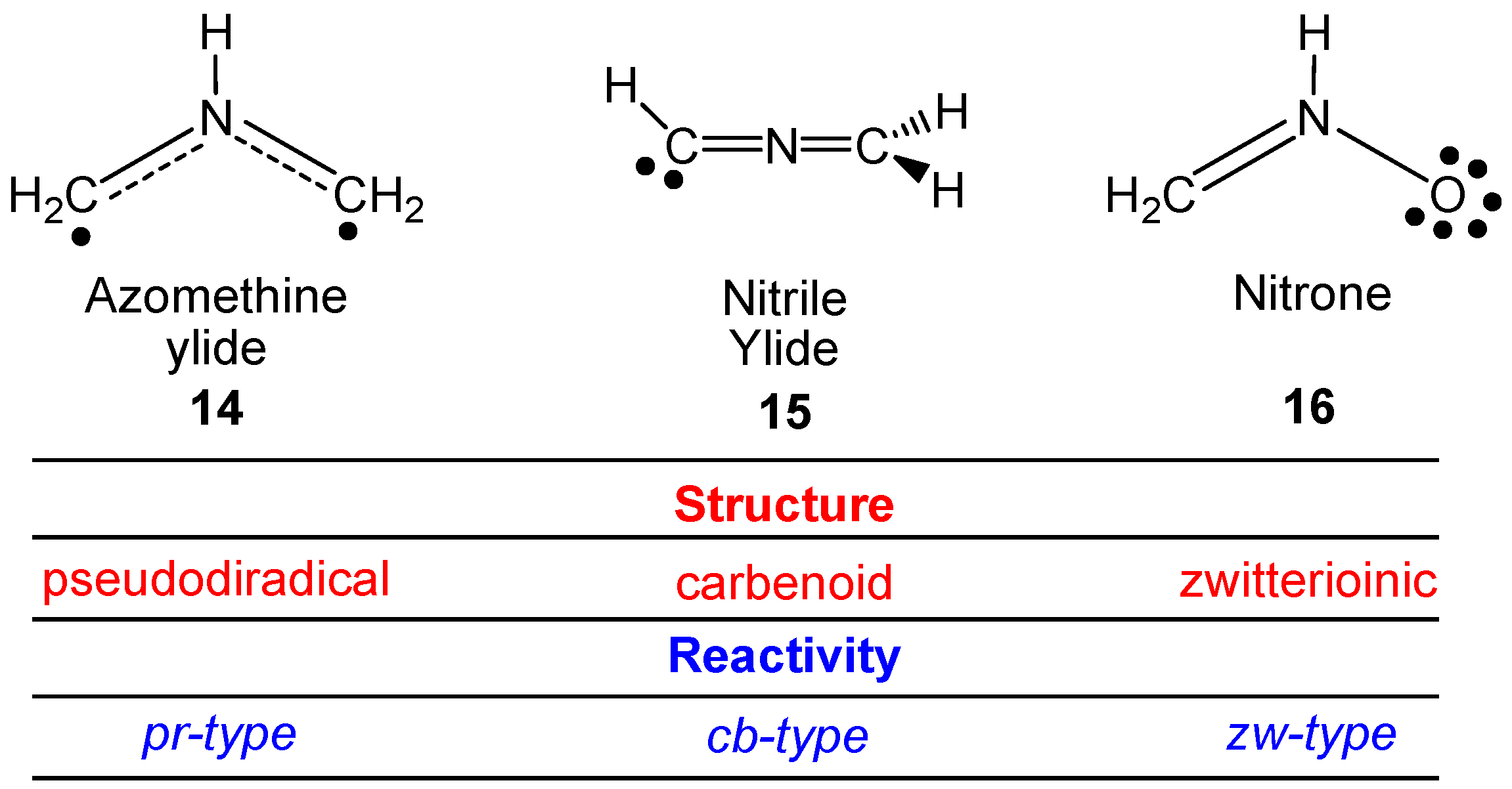
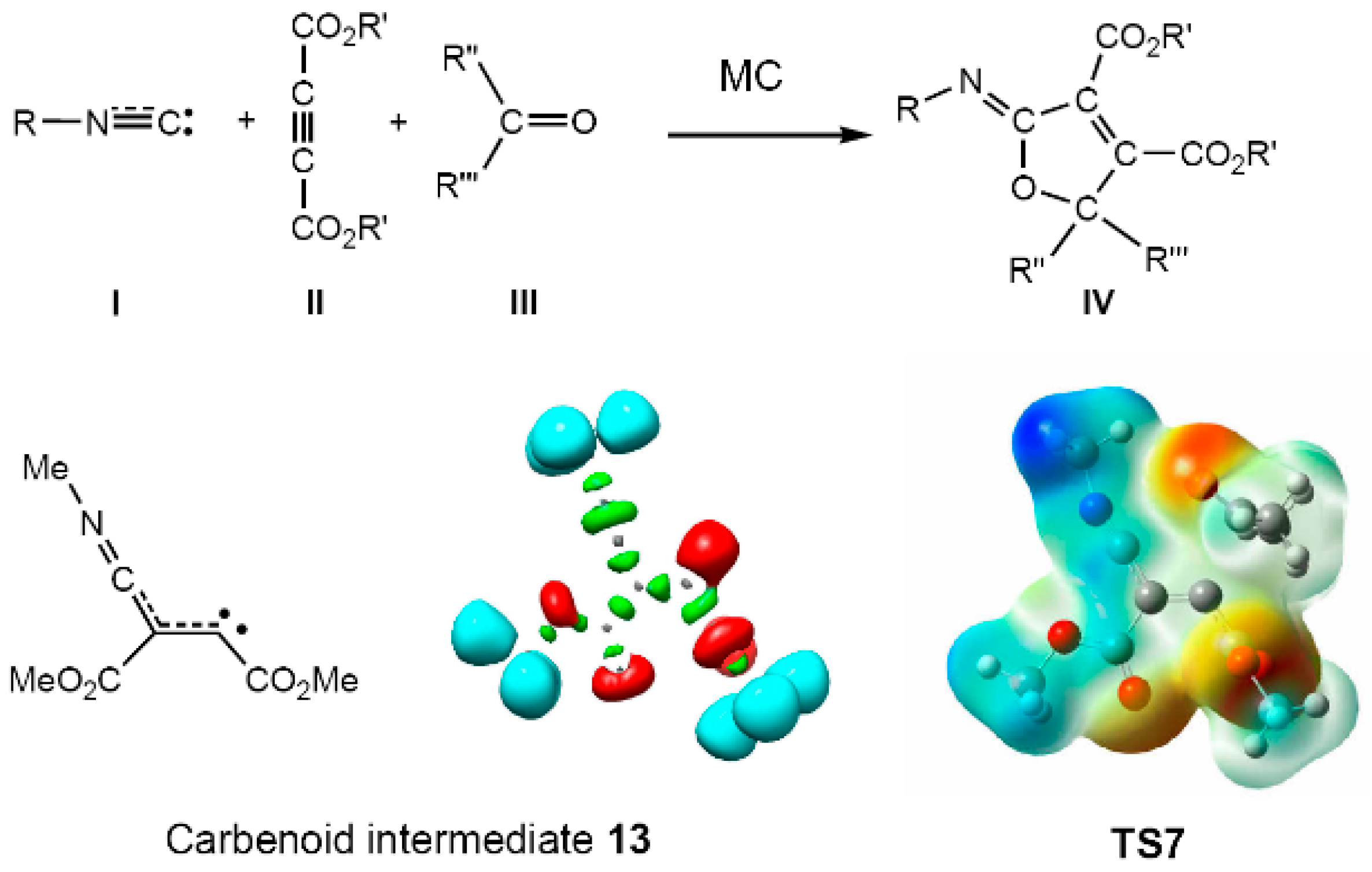
Molecules Free Full Text Molecular Electron Density Theory A Modern View Of Reactivity In Organic Chemistry Html

Electron Density Meaning Youtube
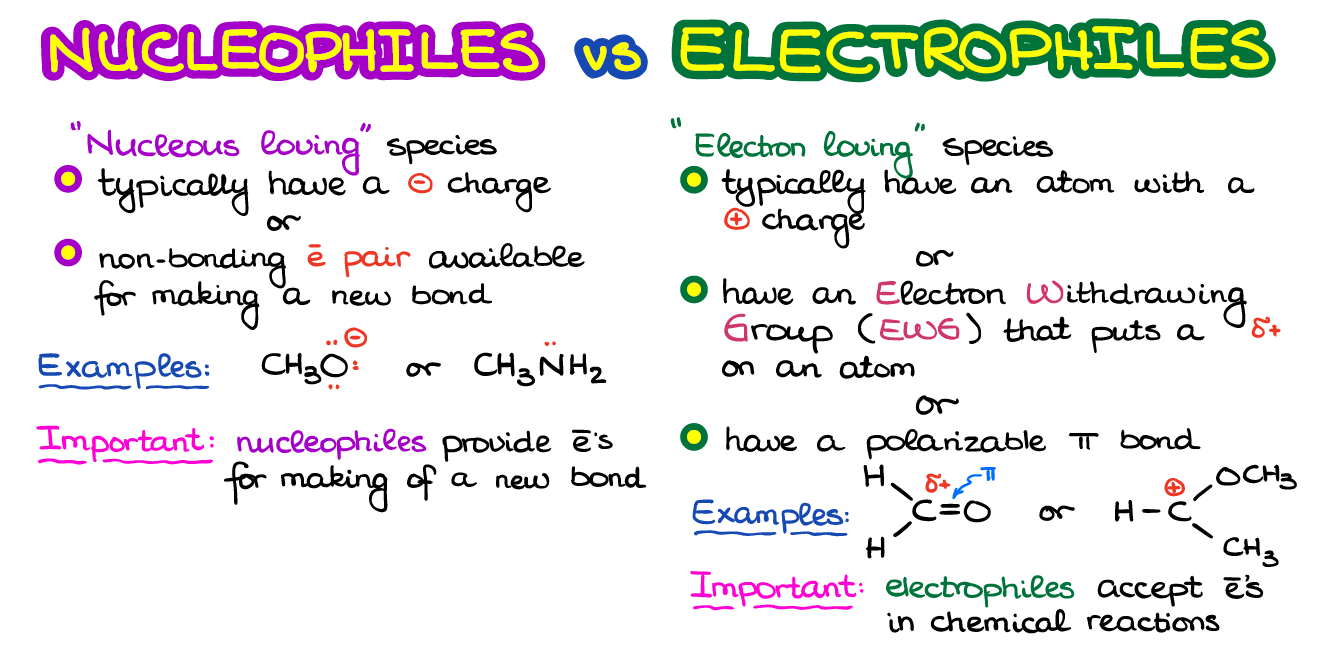
Nucleophiles And Electrophiles Organic Chemistry Tutor

Molecules Free Full Text Molecular Electron Density Theory A Modern View Of Reactivity In Organic Chemistry Html

Electron Density In Benzene Ring Chemistry Stack Exchange
Illustrated Glossary Of Organic Chemistry Term
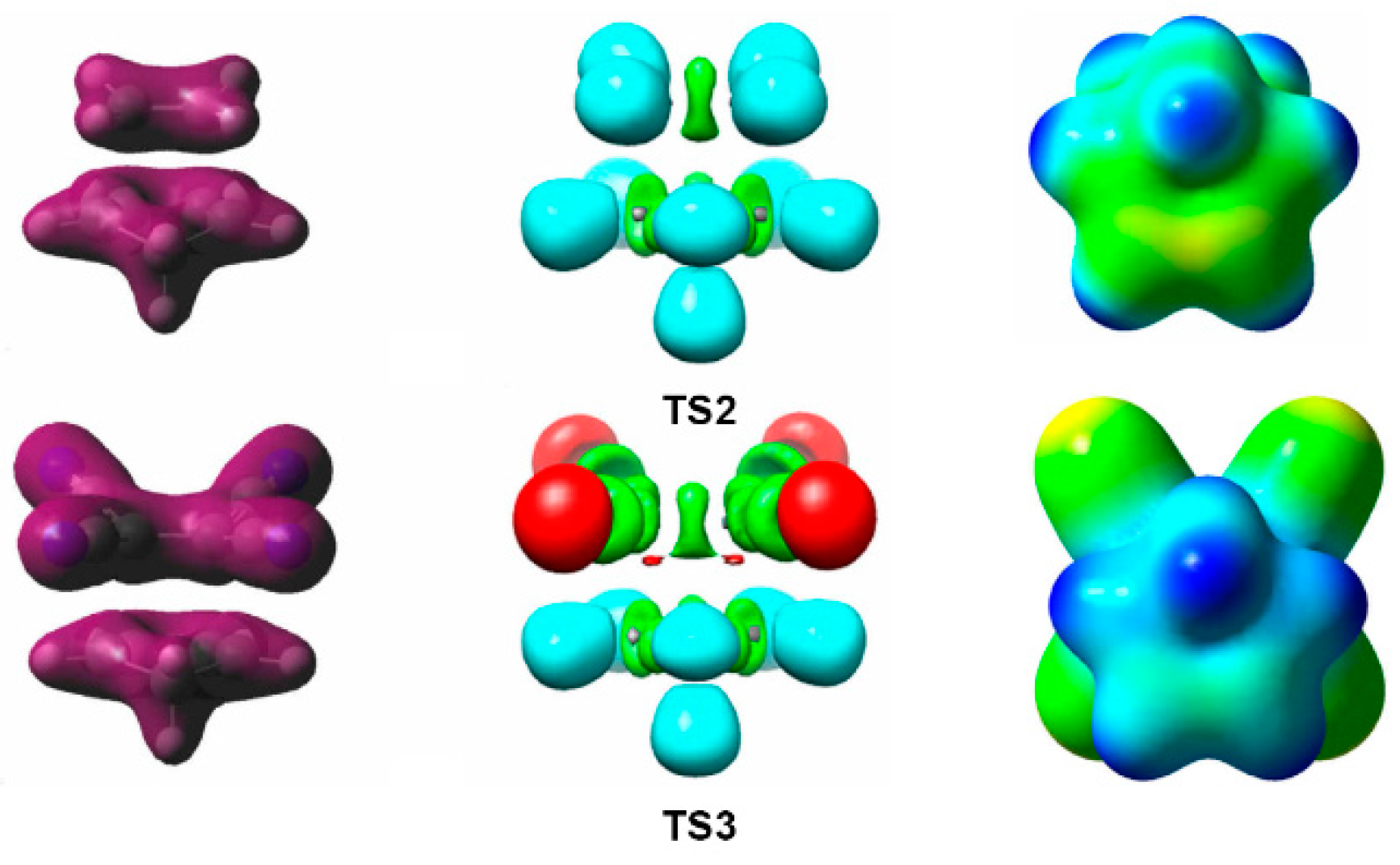
Molecules Free Full Text Molecular Electron Density Theory A Modern View Of Reactivity In Organic Chemistry Html
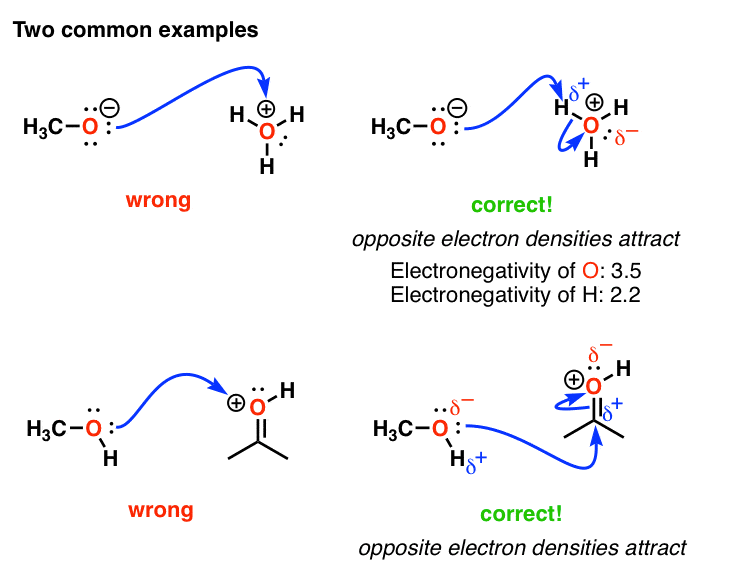
Common Mistakes In Organic Chemistry Formal Charges Can Mislead
Illustrated Glossary Of Organic Chemistry Term

What Is Electron Density Quora
Illustrated Glossary Of Organic Chemistry Term
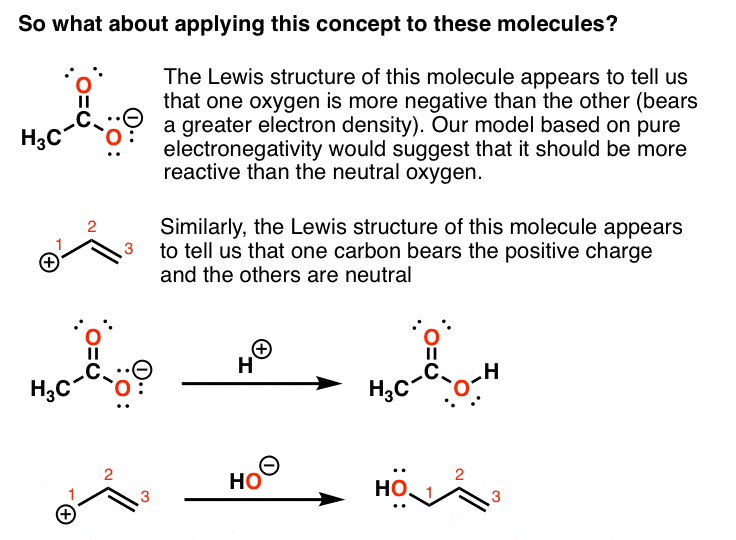
Intro To Resonance In Organic Chemistry Master Organic Chemistry
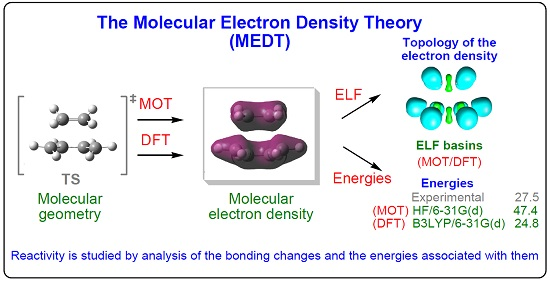
Molecules Free Full Text Molecular Electron Density Theory A Modern View Of Reactivity In Organic Chemistry Html
Illustrated Glossary Of Organic Chemistry Electron Withdrawing Group Ewg
Illustrated Glossary Of Organic Chemistry Term
Illustrated Glossary Of Organic Chemistry Electron Donating Group Edg Electron Releasing Group Erg
Post a Comment for "Definition Of Electron Density In Organic Chemistry"