Definition Of Paramagnetic Atom
An atom that contains any unpaired electrons is known as paramagnetic and an atom with all paired electrons is known as diamagneticDiamagnetic atoms show a weak repulsion to external magnetic fields because the electrons have zero net spin and thus cannot favorably interact with a magnetic field. An atom could have ten diamagnetic electrons but as long as it also has one paramagnetic electron it is still considered a paramagnetic atom.

Electron Configuration And Orbital Diagram Power Point W T Electron Configuration Chemistry Foldables Electrons
The result of a permanent magnetic moment in an atom.

Definition of paramagnetic atom. In other words their constituent atoms or molecules possess a permanent magnetic moment due to the presence of one or more unpaired electrons. Paramagnetism is one of the properties of magnetism. Explanation of Paramagnetism.
An atom that contains any unpaired electrons is known as paramagnetic and an atom with all paired electrons is known as diamagnetic. This is called the fine structure of atomic spectra. This is mainly because the paramagnetic materials have unpaired electrons whereas diamagnetic materials have none of their electrons unpaired.
An atom could have ten diamagnetic electrons but as long as it also has one paramagnetic electron it is still considered a paramagnetic atom. Paramagnetic materials such as aluminum and air have permeabilitys slightly greater than that of free space for air μ. A material aligning itself with the applied field is called paramagnetic material.
If an atom has any unpaired electrons then the material is paramagnetic. In other words any material that possesses atoms with incompletely filled atomic orbitals is paramagnetic. The term paramagnetic refers to the attraction of a material to an external magnetic field while the term diamagnetic refers to the repulsion of a material from an external magnetic field.
A diamagnetic material has a permeability less than that of a vacuum. Consider a substance whose constituent particles contain only. It is a category of magnetism in which materials get weakly attracted by an externally applied magnetic field and form internal or induced magnetic fields in the direction of the magnetic field applied.
Most elements and some compounds are paramagnetic. If it has unpaired electrons then the substance is paramagnetic. An atom is considered paramagnetic if even one orbital has a net spin.
On applying external magnetic field the atomic dipole aligns in the direction of the applied external magnetic field. If all electrons in the particle are paired then the substance made of this particle is diamagnetic. Diamagnetic atoms repel magnetic fields.
Diamagnetic atoms repel magnetic fields. On the other hand if there are only paired electrons the material is diamagnetic. Paramagnetism results from the presence of least one unpaired electron spin in a materials atoms or molecules.
Terms with same n and l quantum numbers are energetially split according to whether the electron spin is parallel or antiparallel to the orbital moment. A simple rule of thumb is used in chemistry to determine whether a particle atom ion or molecule is paramagnetic or diamagnetic. To quantify how paramagnetic a.
Paramagnetism is characteristic of substances whose particles atoms molecules ions or atomic nucleihave intrinsic magnetic moments that in the absence of an external field are oriented randomly with the result that J 0. A paramagnetic electron is an unpaired electron. Diamagnetism is a quantum mechanical effect that is found in all materials but for a substance to be termed diamagnetic it must be the only contribution to the matters magnetic effect.
A paramagnetic electron is an unpaired electron. Paramagnetic materials have some unpaired electrons due to these unpaired electrons the net magnetic moment of all electrons in an atom is not added up to zero. Tell a friend about us add a link to this page or visit the webmasters page for free fun content.
Hence atomic dipole exists in this case. When an external field is applied the magnetic moments of paramagnets atoms are oriented mainly with the field. An atom is considered paramagnetic if even one orbital has a net spin.
Want to thank TFD for its existence. In paramagnetic substances the orbital and spin magnetic moments of atoms are oriented in such a way that each atom has a permanent magnetic dipole moment. However due to thermal motion vibration the direction of the magnetic moments of.
The spin of the unpaired electrons gives them a magnetic dipole moment.

Inert Pair Effect Definition Examples Cause And Consequences Chemsolve Net Electron Configuration Physical And Chemical Properties Chemical Bond

Why Is Cu Diamagnetic While Cu2 Is Paramagnetic Electron Configuration Molecules Air Pollution

Complex Compounds Definition Examples Perfect Imperfect Complex Definition Teaching Chemistry Compound Complex Chemistry Classroom

Gases Consist Of Atoms And Molecules Moving Ceaselessly And Randomly With All Possible Velocities In All Directions Kinetic Theory Thermodynamics Theories
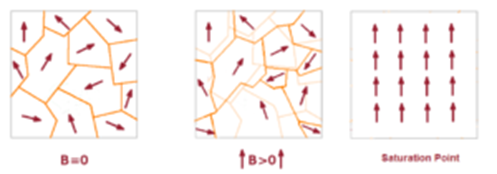
Ferromagnetic Paramagnetic And Diamagnetic Substances

Identifying Elements That Are Paramagnetic Or Diamagnetic Youtube

Paramagnetism And Diamagnetism Video Khan Academy

How Do We Define The Valence Electrons For The Main Chegg Com

Manganese Important Transition Element D And F Block Elements In 2021 Transition Element Organic Chemistry Notes Chemistry Notes

What Is Swarts Reaction And Why Naf Is Not Used In Swarts Reaction Chemsolve Net Reactions Pi Bond Ionic Radius

What Is Evaporation Evaporation Chemistry Molecules

What Is An Atom Definition Of Atom Atom Diagram Atom What Is Atom

Difference Between Carbocation And Carbanion Definition Types Formation Reactions Examples Teaching Chemistry Study Chemistry Chemistry Education

Why Is Ni Nh3 6 Cl2 Paramagnetic But Co Nh3 6 Cl3 Is Diamagnetic Electron Configuration Coordination Number Crystal Field Theory

Is This Paramagnetic Or Diamagnetic Youtube

Actinide Contraction Definition In Chemistry Ionic Radius Electron Configuration Contractions
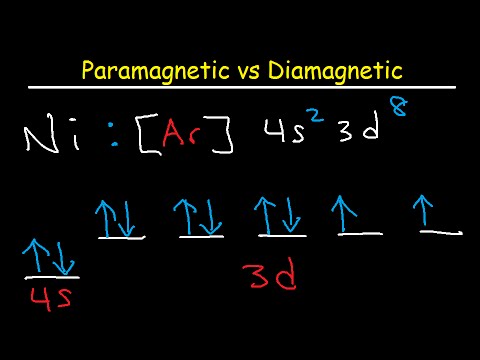
Paramagnetic Vs Diamagnetic Paired Vs Unpaired Electrons Electron Configuration Youtube

Post a Comment for "Definition Of Paramagnetic Atom"