What Is The Operational Definition Of Vaccine
Antibodies are proteins produced naturally by the immune system to fight disease. They also contain other ingredients to keep the vaccine safe and effective.

4 Developing Operational Definitions And Concepts An Assessment Of The Small Business Innovation Research Program Project Methodology The National Academies Press
This term is often used interchangeably with vaccination or inoculation.

What is the operational definition of vaccine. Is influenced by factors such as complacency convenience and confidence. A protein on the surface of an influenza flu virus that can stimulate an immune response. The concept and operational definition of protein epitopes Philos Trans R Soc Lond B Biol Sci.
Vaccine hesitancy is a delay in acceptance or refusal of vaccines despite the availability of vaccine services. These latter ingredients are included in most vaccines and have been used for decades in billions of doses of vaccine. Vaccine hesitancy is complex and context-specific varying across time place and vaccines.
UPDATED March 2019 This list of US. ILI is well established in the literature and in the operational definition of many surveillance databases and its imprecise definition may be inhibiting progress in research and treatment. Recognizes the invading germ such as the virus or bacteria.
A preparation that is administered as by injection to stimulate the bodys immune response against a specific infectious agent or disease. These definitions are operationally defined as testing positive for HCV detection in consecutive visits similar to the definitions considered in the HPV setting. ILI has no role in measuring influenza vaccine effectiveness.
A suspension of attenuated or killed microorganisms viruses bacteria or rickettsiae administered for prevention amelioration or treatment of infectious diseases. Vaccines contain tiny fragments of the disease-causing organism or the blueprints for making the tiny fragments. As a result the body cannot defend itself.
It is influenced by factors such as complacency convenience a fear of needles or a lack of understanding about how vaccines work. It is influenced by factors such as complacency convenience and confidence. The term covers outright refusals to vaccinate delaying.
Definition The separation of a person or group of people known or reasonably believed to be infected with a communicable disease and potentially infectious from those who are not infected to prevent spread of the communicable disease. A vaccine containing partial cellular material as opposed to complete cells. COVID-19 definition is - a mild to severe respiratory illness that is caused by a coronavirus Severe acute respiratory syndrome coronavirus 2 of the genus Betacoronavirus is transmitted chiefly by contact with infectious material such as respiratory droplets or with objects or surfaces contaminated by the causative virus and is characterized especially by fever cough and shortness of.
Vaccine hesitancy is complex and context specific varying across time place and vaccines. Development of synthetic vaccines because it is this type of epitope that should be mimicked by synthesis and used as a vaccine for eliciting protective immunity. Vaccine hesitancy refers to delay in acceptance or refusal of safe vaccines despite availability of vaccination services.
The development of vaccine policies and operational guidelines in English and Hindi one of the 22 scheduled languages of the Republic of India and also one of the official languages of India which is understood spoken and read by more people than English by the technical bodies of MoHFW such as the Immunization Technical Support Unit and the Mission Steering Group was. In this paper we consider several operational definitions of HCV infection that may be used to indicate chronic infections in the context of a vaccine trial. It differs from vaccine effectiveness which measures how well a.
In a special issue of the journal Vaccine guest-edited by WHO and published today experts review the role of vaccine hesitancy in limiting vaccine coverage and explore strategies to address it. Acquired Immune Deficiency Syndrome AIDS. A process by which a person becomes protected against a disease through vaccination.
An antigenic preparation of a typically inactivated or attenuated see attenuated sense 2 pathogenic agent such as a bacterium or virus or one of its components or products such as a protein or toxin a trivalent. Vaccines page lets you view the entire list of US. When you get a vaccine your immune system responds.
Vaccines by name abbreviation trade name or manufacturer. Sage Vaccine Hesitancy Working Group Diagnosing the determinants of vaccine hesitancy in specific subgroups. The Working Group retained the term vaccine rather.
The first demonstration. Vaccine efficacy is the percentage reduction in a disease in a group of people who received a vaccination in a clinical trial. Vaccines and related products sorted by the vaccine name pdf icon 1 page shows the trade name abbreviation manufacturer type eg inactivated and route eg oral.
Anthrax vaccine a cell-free protein extract of cultures of Bacillus anthracis used for immunization against anthrax. Vaccines reduce risks of getting a disease by working with your bodys natural defenses to build protection. The act of introducing a vaccine into the body to produce immunity to a specific disease.
A medical condition where the immune system cannot function properly and protect the body from disease. The Guide to Tailoring Immunization Programmes TIP Butler and MacDonald Journal of Vaccine. The SAGE Working Group on Vaccine Hesitancy concluded that vaccine hesitancy refers to delay in acceptance or refusal of vaccination despite availability of vaccination services.

Advances And Challenges In Vaccine Development And Manufacture Bioprocess Internationalbioprocess International

Operational Definitions Of Child Vaccine Status Download Table

Ri Health Officials Dive Deeper Into Discussion On Phase 2 Vaccine Prioritization Wpri Com
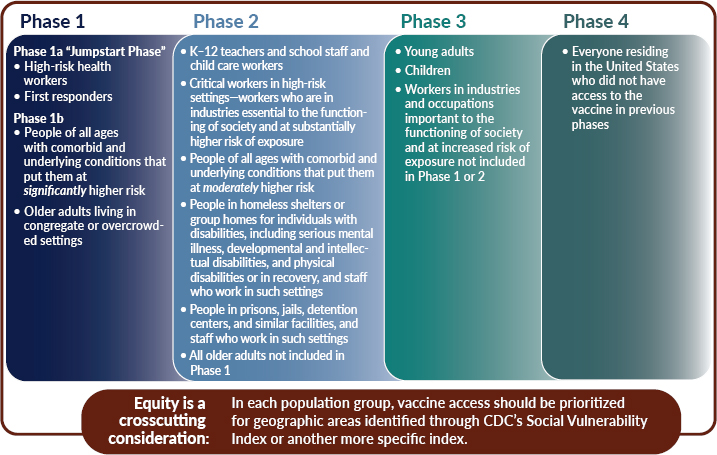
3 A Framework For Equitable Allocation Of Covid 19 Vaccine Framework For Equitable Allocation Of Covid 19 Vaccine The National Academies Press

Operational Definitions Of Child Vaccine Status Download Table

Covid 19 Vaccine Lincoln County Oregon

Operational Definitions Of Child Vaccine Status Download Table
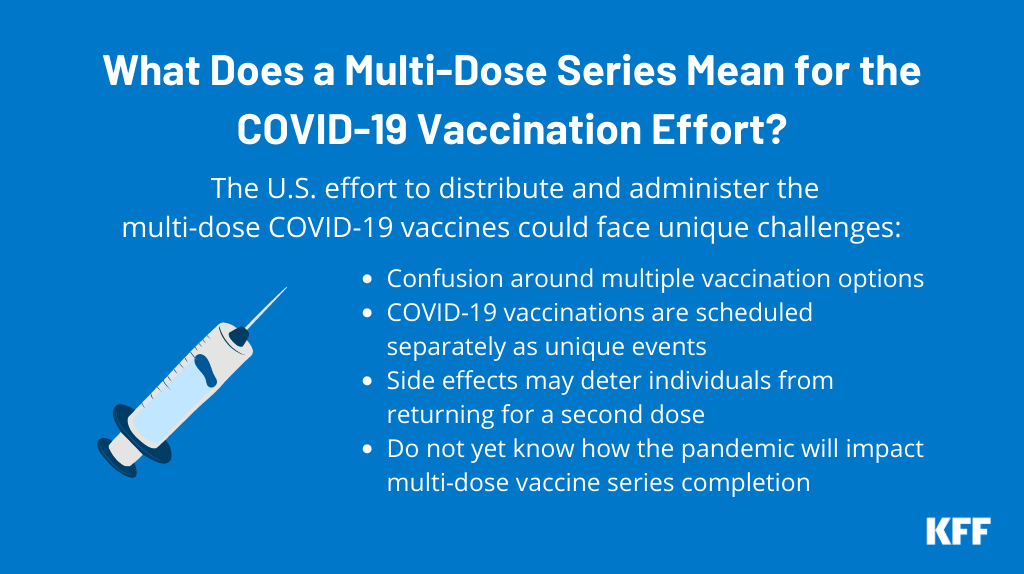
What Does A Multi Dose Series Mean For The Covid 19 Vaccination Effort Kff
What Are Viral Vector Based Vaccines And How Could They Be Used Against Covid 19 Gavi The Vaccine Alliance

Operational Definition In Relation To Who National Vaccination Schedule Download Scientific Diagram

Advances And Challenges In Vaccine Development And Manufacture Bioprocess Internationalbioprocess International
Https Apps Who Int Iris Bitstream Handle 10665 338855 Who Euro 2021 1867 41618 56856 Eng Pdf Sequence 1 Isallowed Y
Https Www Who Int Immunization Sage Meetings 2012 November 4 Indicators Spreadsheets Pdf Ua 1

Operational Definition In Relation To Who National Vaccination Schedule Download Scientific Diagram
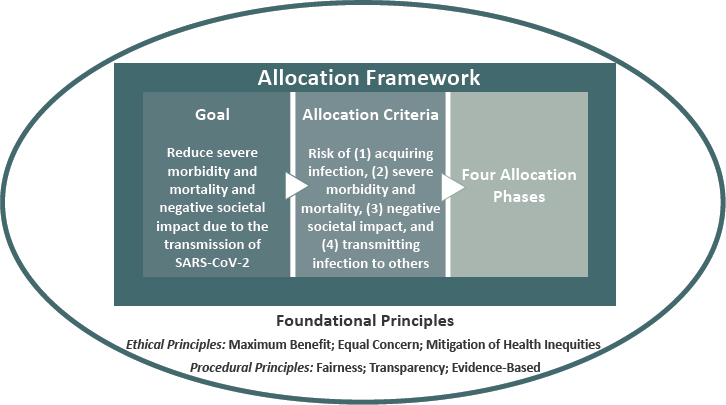
3 A Framework For Equitable Allocation Of Covid 19 Vaccine Framework For Equitable Allocation Of Covid 19 Vaccine The National Academies Press

Operational Definitions Of Child Vaccine Status Download Table
Https Www Doh Wa Gov Portals 1 Documents Pubs 348 269 Glossaryimmunizationpublichealthterms Pdf
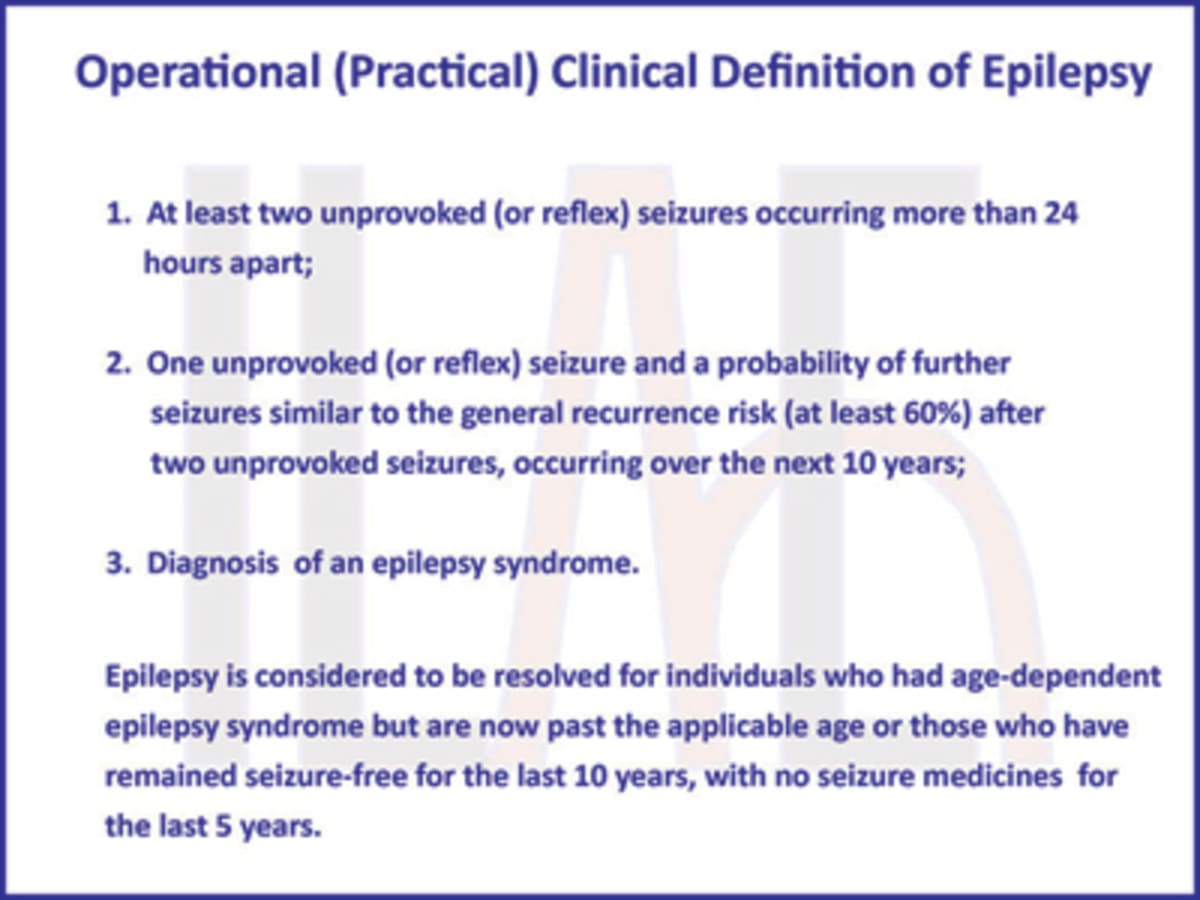
Definition Of Epilepsy 2014 International League Against Epilepsy
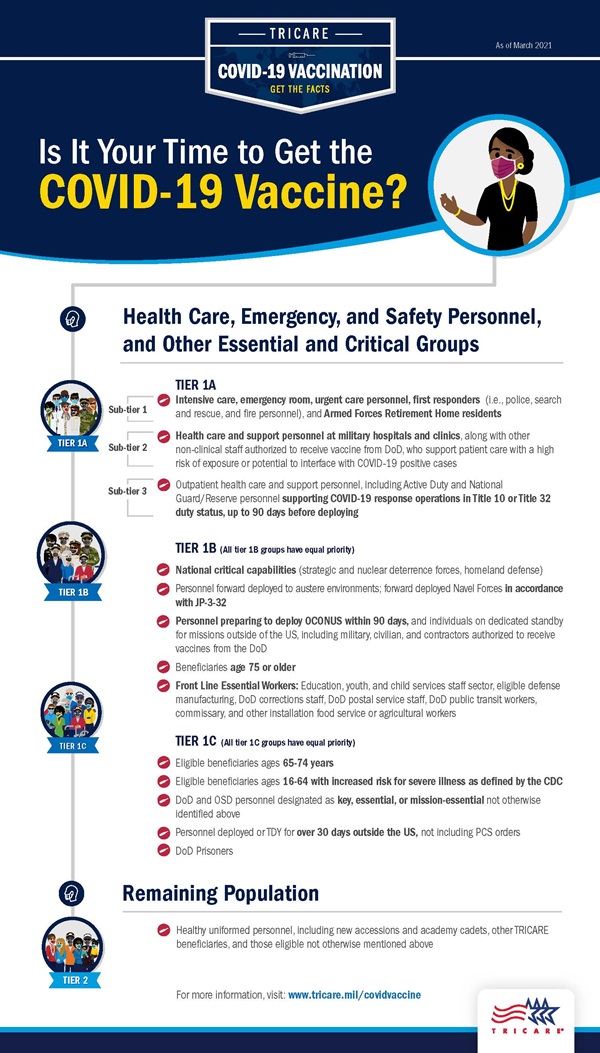
Post a Comment for "What Is The Operational Definition Of Vaccine"