Definition Of Gas Evolution Reactions
For example H2CO3 readily decomposes into H2O and CO2 gas H2CO3aq H2Ol CO2g. A gas evolution reaction is a chemical reaction in which one of the end products is a gas such as oxygen or carbon dioxide.
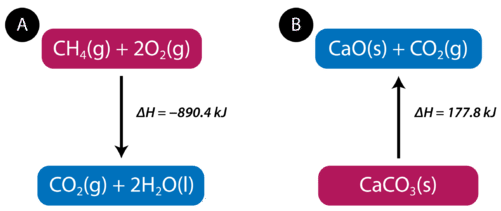
8 7 Enthalpy Change Is A Measure Of The Heat Evolved Or Absorbed Chemistry Libretexts
A gas-evolution reaction is one in which heat is produced upon mixing two.

Definition of gas evolution reactions. Another way gases can form in solution is through the decomposition of weak electrolytes. A common indicator used to detect the presence of carbon dioxide gas. Carbon dioxide enters the atmosphere through burning fossil fuels coal natural gas and oil solid waste trees and other biological materials and also as a result of certain chemical reactions eg manufacture of cementCarbon dioxide is removed from the atmosphere or sequestered when it is absorbed by plants as part of the biological carbon cycle.
The H ag from the acid combines with the OH- from the base to form HO1 A gas-evolution reaction is an aqueous reaction that forms a gas upon mixing two solutions. If you are having trouble with Chemistry Organic Physics Calculus or Statistics we got your back. What Does Hydrogen Evolution Reaction Mean.
A gas-evolution reaction is one in which a solid or precipitate forms upon mixing two solutions. In the following examples an acid reacts with a carbonate producing salt carbon dioxide and water respectively. 2 C 4 H 10 g 13 O 2 g Æ 8 CO 2 g 10 H 2 O l combustion b.
Our videos will help you understand concepts solve your homework and do great on your exams. A gas-evolution reaction an acid and base are mixed. Carbon dioxide CO 2.
When relatively pure oxygen is required industrially it is isolated by distillation of liquified air. GAS ABSORPTION DESORPTION. It may be due to the fact that the solubility of a gas is less in the solid and liquid metal respectively as when hydrogen is evolved by aluminum and its alloys or to the promotion of a gas-forming reaction as when iron oxide and.
Our videos prepare you to succeed in your college classes. Hydrogen evolution usually occurs on metals such as. A hydrogen evolution reaction is the production of hydrogen through the process of water electrolysis.
Lowry 18741936 who defined acidbase reactions in terms of the transfer of a proton H ion from one. Give the type of reaction for each of the following as either an acid-base combustion gas evolution or precipitation reaction. For example nitric acid reacts with sodium carbonate to form sodium nitrate carbon dioxide and water Table PageIndex1.
A chemical process that produce a gas such as oxygen or carbon dioxide limewater. H2S is just one example of a gaseous substance that can form in a solution reaction. Used to determine if the reaction of a substance with water or the humid air involves the evolution of a dangerous quantity of a gas or several gases likely to be very flammable.
Terms in this set 4 Reactant Type. Oxygen evolution is the process of generating molecular oxygen O 2 by a chemical reaction usually from waterOxygen evolution from water is effected by oxygenic photosynthesis electrolysis of water and thermal decomposition of various oxides. NH3aqH2Ol NH4aqOHaq NH 3 a q H 2 O l NH 4 a q OH a q This is by definition an acid-base reaction in this case involving the transfer of H ions from water molecules to ammonia molecules.
Gas evolution reactions may be carried out in a fume chamber when the gases produced are poisonous when inhaled or explosive. The biological process supports aerobic life. This set contains tabular data pertaining to the reactant types intermediate products gasses evolved and examples with respect to gas-evolution reactions.
An aqueous solution of CaOH2. Definition of gas evolution The liberation of gas in the form of bubbles during the solidification of metals. Under typical conditions only about.
The evolution of hydrogen is possibly limited and based on the desorbing of molecules coming from the cathode surface. Gas absorption also known as scrubbing is an operation in which a gas mixture is contacted with a liquid for the purpose of preferentially dissolving one or more components of the gas mixture and to provide a solution of them in the liquid. Therefore we can see that there is a mass transfer of the component of the gas from the gas phase to the liquid phase.
Because of the limitations of the Arrhenius definition a more general definition of acids and bases was needed. Let us help you simplify your studying. A gas evolution reaction is a chemical process that produces a gas such as oxygen or carbon dioxide.
One was proposed independently in 1923 by the Danish chemist J. Brønsted 18791947 and the British chemist T.

Introduction To Double Replacement Reactions Youtube

Oxygen Evolution Reaction An Overview Sciencedirect Topics

7 8 Acid Base And Gas Evolution Reactions Chemistry Libretexts
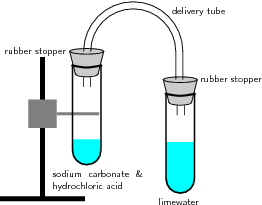
7 8 Acid Base And Gas Evolution Reactions Chemistry Libretexts

Hydrogen Evolution Reaction An Overview Sciencedirect Topics

Gas Evolution Reaction Definition And Examples

Ib Chemistry Notes On Kinetics Sl Rates Of Reaction

Thermal Explosion An Overview Sciencedirect Topics
Which Neutral Gas Is Evolved When Lead Nitrate Undergoes Thermal Decomposition Quora

Types Of Arrows Used In Chemistry Curlyarrows Chemistry Tutorials

Gas Evolution Reactions Definition Examples Video Lesson Transcript Study Com

Chemistry Lesson Gas Evolution Reactions Youtube
Https Www Cell Com Joule Pdf S2542 4351 20 30032 5 Pdf

Gas Evolution Reaction Definition And Examples
Types Of Chemical Reactions Youtube

Hydrogen Evolution Reaction An Overview Sciencedirect Topics

Chemistry Lesson Gas Evolution Reactions Youtube

Gas Evolution Reactions Definition Examples Video Lesson Transcript Study Com

Ib Chemistry Notes On Kinetics Sl Rates Of Reaction
Post a Comment for "Definition Of Gas Evolution Reactions"