Atom Definition Biology Example
In electrically neutral atoms the number of protons equals the number of electrons. Examples include neon hydrogen and argon.

Atoms Definition Overview Expii
A neutral atom has an equal number of protons and electrons so that the positive and negative charges exactly balance.

Atom definition biology example. Basically any material with a composition that includes more than one element symbol or that has a subscript following an element symbol is a molecule or compound rather than an atom. For example one gold atom has all of the properties of gold in that it is a solid metal at room temperature. Atoms are extremely small and are made up of a few even smaller particles.
Sodium Na Oxygen O Carbon C Atomic model They have been modeled to be like a solar system in miniature. Bit I dont believe theres an atom of meaning in it Lewis Carroll Alices Adventures in Wonderland. It consists of one proton and one electron.
There are electrons which revolve around a nucleus. Examples of substances that are not atoms include water H 2 O table salt NaCl and ozone O 3. A simple example is that of say sodium fluoride.
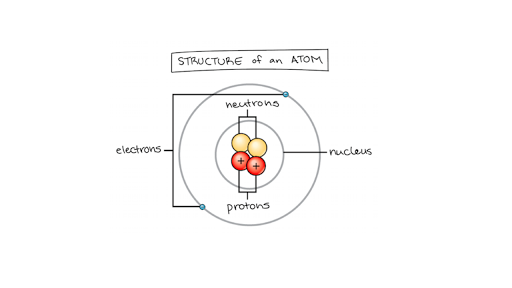
An atom is the smallest unit of matter that retains all of the chemical properties of an element. While its name originally referred to a particle that couldnt be divided any morethe smallest thing possiblewe now know that each atom is generally made up of smaller particles. The smallest unit of an element consisting of at least one proton and for all elements except hydrogen one or more neutrons in a dense central nucleus surrounded by one or more shells of electrons.
An atom is the smallest unit of matter that retains all of the chemical properties of an element. For example the atom isnt an elementary particle. The simplest forces between atoms are those which arise as a result of electron transfer.
The atom is the basic building block for all matter in the universe. The basic particles that make up an atom are electrons protons and neutrons. The sodium atom has a nuclear charge of 11 with 2 electrons in the K shell 8 in the L shell and 1 in the M shell.
A tiny particle. The single most important characteristic of an atom is its atomic number usually denoted by the letter Z which is defined as the number of units of positive charge protons in the nucleus. An atom is the smallest unit of matter that retains all of the chemical properties of an element.
The fluorine atom has a nuclear charge of 9 with 2 electrons in the K shell and 7 in the L shell. Atoms fit together with other atoms to make up matter. All the matter in this universe is made of tiny particles called atoms.
For example if an atom has a Z of 6 it is carbon while a Z of 92 corresponds to uranium. An Atom is the smallest particle that something can be broken down into and still retain the original chemical properes. For example a gold coin is simply a very large number of gold atoms molded into the shape of a coin with small amounts of other contaminating elements.
An elementary particle is a particle that is the smallest and most basic component of matter and cannot be broken down into smaller particles. For example Gold is made of only gold atoms. Anything that has a massin other words anything that occupies spaceis composed of atoms.
Atoms are the main building blocks of matter. An example of an atom is a single particle of any element on the periodic table. The atom is considered the basic building block of matter.
For example chair water air plants and humans etc. Atoms are fundamental units of matter that cannot be broken down by any chemical means. There are 92 different kinds of atoms in nature and are listed in the periodic table too.
The atomic mass for an atom of hydrogen is one dalton which is calculated. Atoms combine to form molecules which then interact to form solids gases or liquids. For example water is composed of hydrogen and oxygen atoms that have combined to form water molecules.
The smallest unit of an element consisting of at least one proton and for all elements except hydrogen one or more neutrons in a dense central nucleus surrounded by one or more shells of electrons. Examples of Atomic Mass Hydrogen is the lightest naturally occurring element. All the materials are made up of very small particles called atoms.
The smallest particle of an element that can exist alone or in combination carbon atoms.

The Building Blocks Of Molecules Biology I

What Is Atom Definition With Example Class 9 Chemistry Structure Of Atom In Urdu Hindi Youtube

Atoms Definition Overview Expii

Elements Atoms Molecules Ions Ionic And Molecular Compounds Cations Vs Anions Chemistry Youtube

Atoms Biology For Non Majors I

What Are Atoms Molecules Definition Differences Video Lesson Transcript Study Com

What Is An Atomic Number Definition And Examples
Matter Elements And Atoms Chemistry Of Life Article Khan Academy

What Is An Atom Lesson For Kids Video Lesson Transcript Study Com

Atomic Structure Electrons Protons Neutrons And Atomic Models

Atoms Definition Overview Expii

Atomic Mass Videos Concepts Calculations Methods Examples

Atomic Number Atomic Mass And Isotopes Article Khan Academy
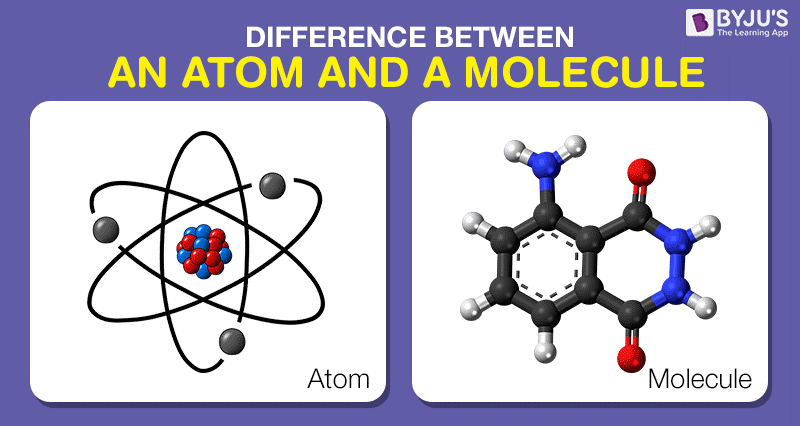
Difference Between Atom And Molecule In Tabular Form

Molecule Definition Examples Structures Facts Britannica

Introduction To Structure Of Atom Proton Neutron Electron With Examples
Molecule Definition Types And Examples Biology Dictionary

Atoms And Elements Biology For Non Majors I

Post a Comment for "Atom Definition Biology Example"