Definition Of Mass Defect In Physics
The missing mass is the energy released by the formation of the atomic nucleus. So that means you would have to put in energy to seperate the atom since both of the products require more.
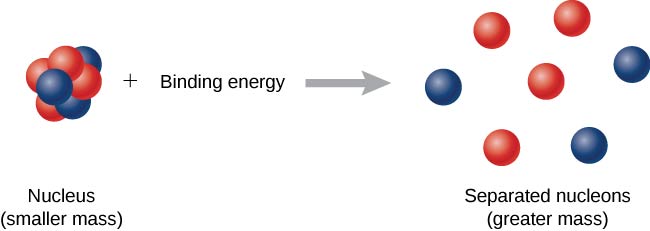
10 3 Nuclear Binding Energy Physics Libretexts
It is equal to the mass defect 2.

Definition of mass defect in physics. This mass is typically associated with the binding energy between nucleons. Mass defect is associated with the binding energy of the nucleus. 221 The difference in mass Δ M is called the mass defect.
General Physics the energy that must be supplied to a stable nucleus before it can undergo fission. Mass defect has also entered into the mass spectrometry terminology with the availability of high resolution mass spectrometry and has found application in mass spectral analysis. The actual atomic mass is less than the predicted mass calculated by adding the masses of nucleons.
The difference between the mass of the atom and the sum of the masses of its parts is called the mass defect Δm. In physics and chemistry a mass defect refers to the difference in mass between an atom and the sum of the masses of the protons neutrons and electrons of the atom. American Heritage Dictionary of the English Language Fifth Edition.
Im assuming that mass is converted to energy and the mass defect will increase. Also called mass deficiency. A deuteron having a mass defect of 000239 u has.
Change equating mass to quantity of matter is totally untenable2 When attracting particles come together to form a bound systemfor example an atom or a nucleus or even a planet16the mass of the whole M will be less than the sum of the masses of the constituents. Mass defect is known as the mass difference which means the sum of the total mass of the nucleus constituents. The nuclear binding energy accounts for the noticeable difference between the actual mass of atoms nucleus and.
It is a fundamental property of the nucleus and the principle behind nuclear energy. The mass defect is given by the formula. In nuclear physics the mass defect is the difference in the mass of a composite particle and the sum of the masses of its component parts.
The protons and neutrons are more than that of the mass of the nucleus. In mass spectrometry the mass defect is defined as the difference between the exact mass and the nearest integer mass. In special theory of relativity certain types of matter may be created or destroyed but in all of these processes the mass and energy associated with such matter remains unchanged in quantity.
Irving Kaplan of MIT in Nuclear Physics 2nd edition 1962 pg. Mass defect which is the mass missing in the resulting nucleus represents the energy released during formation of nucleus. Mass Defect and Binding Energy Careful measurements have shown that the mass of a particular atom is always slightly less than the sum of the masses of the individual neutrons protons and electrons of which the atom consists.
When Nuclear fission of uranium occurs the binding energy of the two products is higher and so the overall binding energy is higher than it was before. A nuclide AX has N neutrons and Z protons so that the difference in mass is derived as m Zmp Nmn mtot. Krane Introductory to Nuclear Physics defines mass defect as Δ m AZ-A c 2 where m is the mass of the nucleus with atomic number Z and mass number A and he says that given Δ we can use the nuclear binding energy to deduce the.
Mass defect in American English Physics the difference between the mass of an atom and the sum of the masses of the individual neutrons and protons in its nucleus expressed in atomic mass units. Thus BE m c2 Zmp Nmn mtotc2. Mass defect definition the amount by which the mass of an atomic nucleus differs from the sum of the masses of its constituent particles being the mass equivalent of the energy released in the formation of the nucleus.
The amount by which the mass of an atomic nucleus is less than the sum of the masses of its constituent particles. This additional mass is accounted for by binding energy that is released when a nucleus is formed. In atomic mass The difference called the mass defect is accounted for during the combination of these particles by conversion into binding energy according to an equation in which the energy E released equals the product of the mass m consumed and the square of the velocity of light in vacuum c.
It is the amount of mass which would be converted to energy if a particular atom were to be assembled from the requisite number of protons neutrons and electrons. Here m n m p -represent combined mass of proton and neutron and m o - denotes original mass of atom. Mass defect is the difference between the actual atomic mass and the predicted mass calculated by adding the mass of protons and neutrons present in the nucleus.
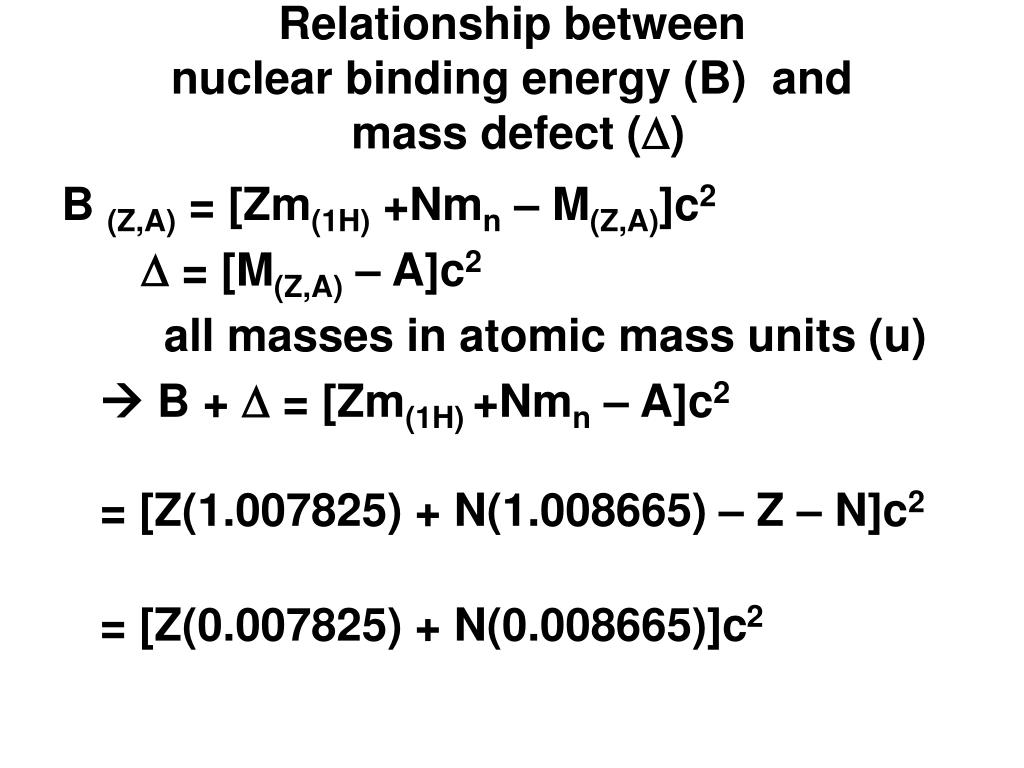
Ppt Relationship Between Nuclear Binding Energy B And Mass Defect D Powerpoint Presentation Id 699522

Mass Defect And Binding Energy Video Khan Academy

Properties Of Nucleus 26 2 Binding Energy And Mass Defect Unit 26 Nucleus Is Defined As The Central Core Of An Atom That Is Positively Charged Ppt Download

Mass Defect Binding Energy 1 Of 7 An Explanation Youtube

Mass Defect And Binding Energy
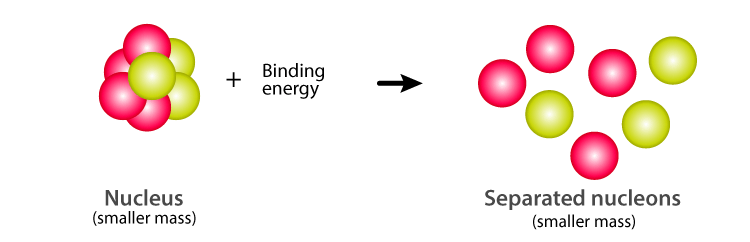
Nuclear Binding Energy Definition Formula Explanation
Difference Between Binding Energy And Mass Defect

Lecture 1 2 C 2015 Calculate The Mass Defect And The Binding Energy Per Nucleon For A Particular Isotope Calculate The Mass Defect And The Binding Ppt Download

Important Questions For Cbse Class 12 Physics Mass Defect And Binding Energy

What Is The Formula For Mass Defect Quora
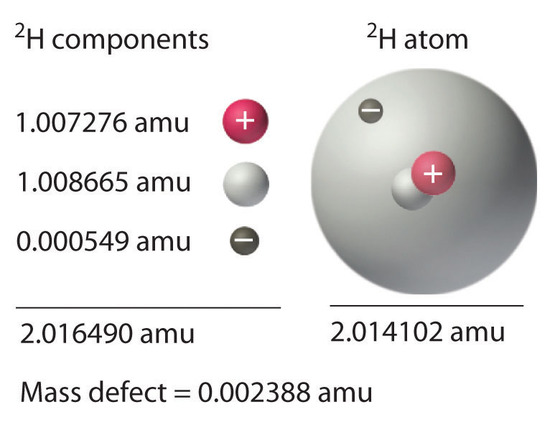
21 6 Energy Changes In Nuclear Reactions Chemistry Libretexts
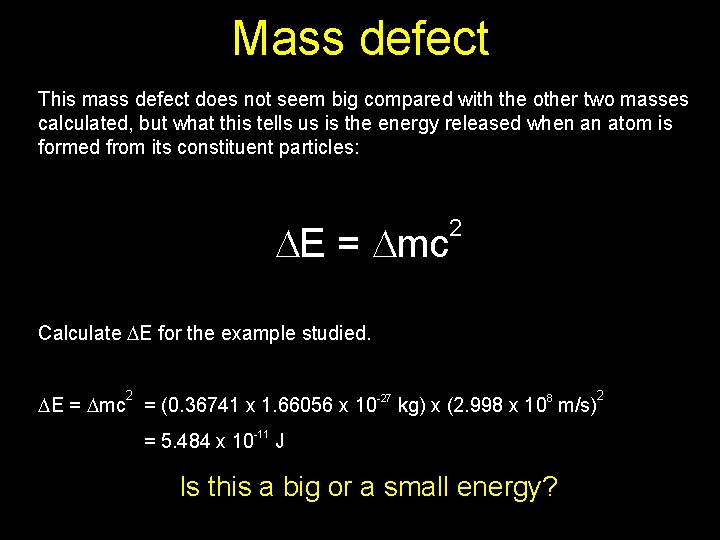
Isotopes Mass Defect E 2 Mc Isotopes Thanks

Nuclear Binding Energy Per Nucleon Mass Defect Problems Nuclear Chemistry Youtube
What Is The Formula For Mass Defect Quora
Define Mass Defect Of A Nucleus Binding Energy Of 8 O 16 Is 127 5mev Write The Value Of Its Binding Energy Per Nucleon Write The Value Of 1ev Energy In Joule

Properties Of Nucleus 26 2 Binding Energy And Mass Defect Unit 26 Nucleus Is Defined As The Central Core Of An Atom That Is Positively Charged Ppt Download

Mass Defect And Binding Energy Ib Physics Youtube

A Nucleus Is More Than Just Mass Ppt Video Online Download
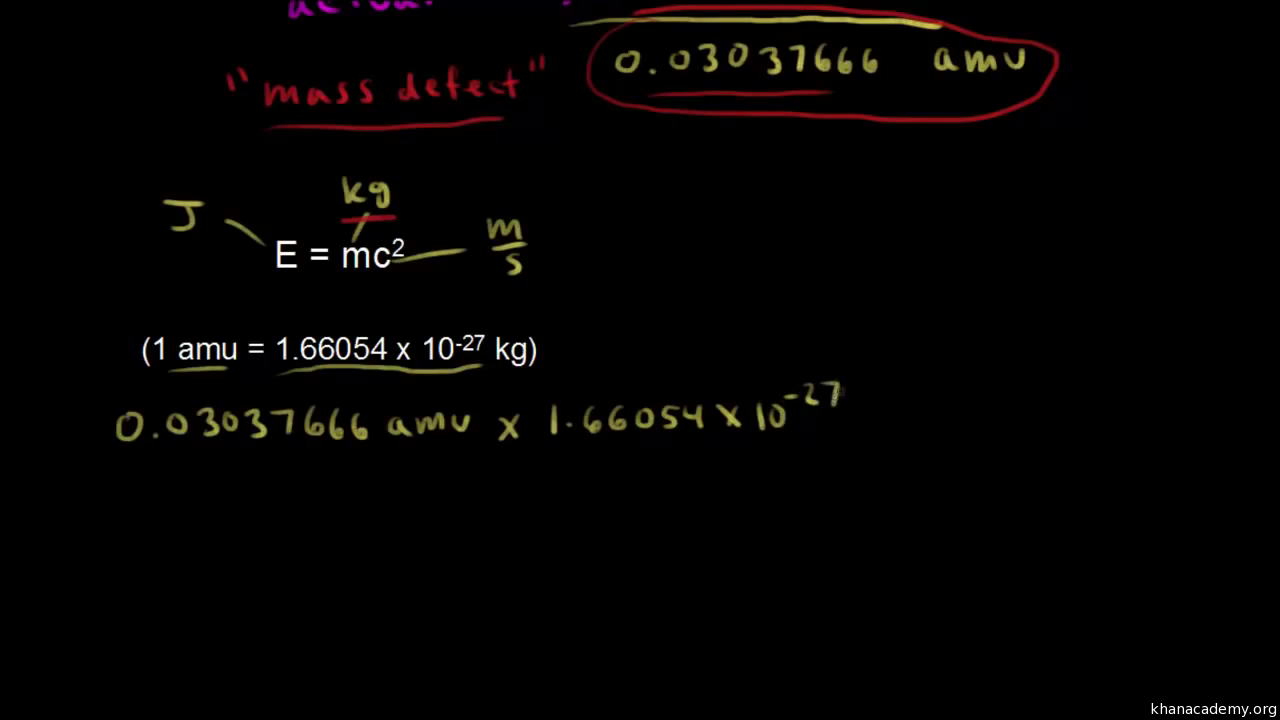
Mass Defect And Binding Energy Video Khan Academy

Post a Comment for "Definition Of Mass Defect In Physics"