Definition Atom Ground State
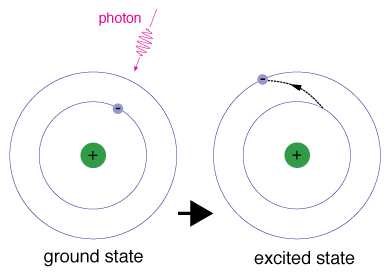
This means that there is one unpaired electron and the total electron spin of the atom is 12. Excited state the condition of a nucleus atom or molecule produced by the addition of energy to the system as the result of absorption of photons or of inelastic collisions with other particles or systems.
Ground state refers to the state in which all electrons in a system an atom molecule or ion are in the lowest possible energy levels.
Definition atom ground state. Therefore the ground state is known to have no energy when compared to an excited state because the electrons are in a zero energy level. In chemistry and physics the ground state is defined as the lowest allowed energy state of an atom molecule or ion. A valence electron can be promoted to an orbit even farther from the.
Ground state electron configurations are the foundation for understanding molecular bonding properties and structures. For example an electron in the lowest energy orbital in a hydrogen atom is in a ground state. In chemistry of atoms and molecules are important as are their excited states.
Normally an electron occupies the energy level called the ground state. In the definition of the ground state of an atom it is given that The ground state is one in which the electrostatic energy of attraction is minimum. This means that all the electrons in a ground state atom are in the lowest energy levels possible.
This state is called the ground state of the atom. From the electrons in an atom to the differing orbitals and hybridization the ground state electron configuration sheds light on many different atomic properties. Consider a carbon atom whose electron configuration is.
Ground state the condition of lowest energy of a nucleus atom or molecule. Higher energy states are described as excited states. Ground state The state of lowest possible energy especially of subatomic particles.
The ground state is also called the vacuum state. A ground state atom possesses electrons in its lowest energy orbitals. The meaning of the preceding definition is as follows.
An atom is in a ground state when all of the electrons in an atom are at their lowest energy levels. The ground state applies to any quantized property of a particle. It can move back to the ground state from an excited state by releasing the extra energy.
Ground State Atom Definition. The atom in which electrons cannot lower their total energy by the transfer of one or more than one electron to other different orbitals is known as a ground state atom. The caesium atom has a ground state electron state with configuration Xe 6s 1 and consequently atomic term symbol 2 S 12.
A ground-state atom is an atom in which the total energy of the electrons can not be lowered by transferring one or more electrons to different orbitals. The state of a physical system having the lowest possible potential energy. If there is more than one possible ground state degenerate states are said to exist.
In other words the ground state represents the most stable configuration. Ground state definition is - the state of a physical system as of an atomic nucleus or an atom having the least energy of all the possible states called also ground level. But when the atom is in its ground state then the electron and the nucleus will be closest as compared to when they are in the excited state.
The ground state of a. In an excited state electrons spread out to higher energy levels and not all are in their lowest levels. The ground state electron configuration is the arrangement of electrons around the nucleus of an atom with lower energy levels.
Moreover the nucleus of caesium-133 has a nuclear spin equal to 72. When subatomic particles such as electrons and neutrons are at a higher level of energy than their ground states they emit radiation. State of lowest energy ground state in which the electrons systematically fill all the orbits from those nearest the nucleus outward to some larger orbit containing the outermost valence electrons.
The American Heritage Student Science Dictionary Second Edition. That is in a ground-state atom all electrons are in the lowest possible energy levels. When the electron moves to a higher level it is called the excited state.
The ground state of an atomic nucleus atom or molecule is its lowest energy state. A state of a physical system such as an atomic nucleus an atom or a molecule that is higher in energy than the ground state Examples of excited state in a Sentence Recent Examples on the Web The system transitions by passing through a mixture of the excited state and ground state a quantum phenomenon known as superposition.
Difference Between Ground State And Excited State Definition Characteristics And Differences

Ground State Vs Excited State Electron Configuration Example Practice Problems Explained Summary Youtube
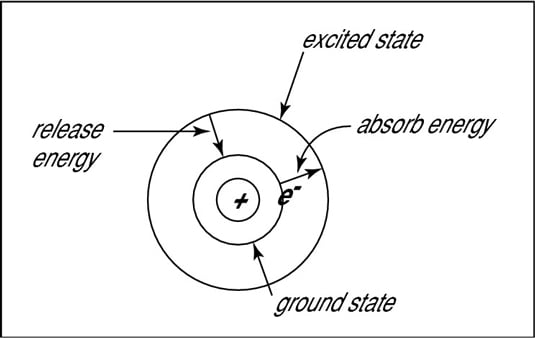
Atomic Structure The Bohr Model Dummies
Definition Of Ground State Chemistry Dictionary
Difference Between Ground State And Excited State Definition Characteristics And Differences

Ground State Vs Excited State Of An Atom A Definitive Analysis Science Struck

Ground State Vs Excited State Of An Atom A Definitive Analysis Science Struck

Spectroscopy Of Electronically Excited States

6 Excited State Vs Ground State Youtube

Ground State Vs Excited State Of An Atom A Definitive Analysis Science Struck
What Is The Ionization Energy Of Rubidium Atoms In Kilojoules Per Mole If Light With L 58 4 Nm Produces Electrons With A Velocity Of 2 450 10 6 M S 2 450 10 6 M S Socratic
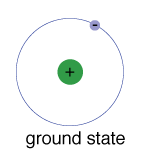

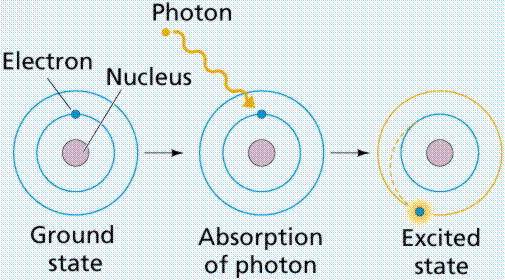
Post a Comment for "Definition Atom Ground State"