Definition Of Polar Molecule Water
Thus droplets or beads of water form on a nonpolar surface because water molecules adhere together instead of adhering to the surface. Molecules form when two or more atoms form chemical bonds with each other.
Polar And Non Polar Molecules Vce Chemistry
These nonpolar molecules do not like to mix with water a very polar molecule.
Definition of polar molecule water. In such a bond there is a charge separation with one atom being slightly more positive and the other more negative ie the bond will produce a dipole moment. It doesnt matter if the atoms are the same or are different from each other. Some chemical species such as chains of carbon molecules share.
In contrast a non-polar. Examples of Polar Molecules. In a b the polar covalent bonds are shown as lines.
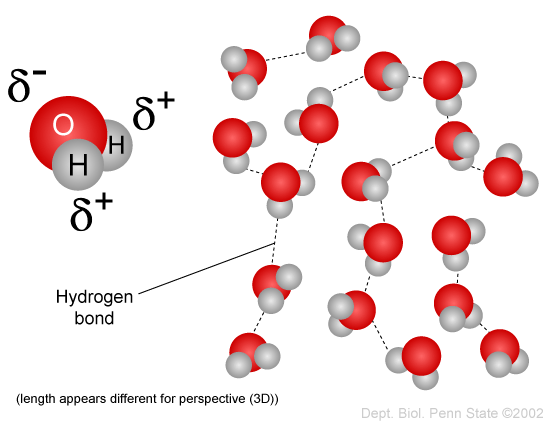
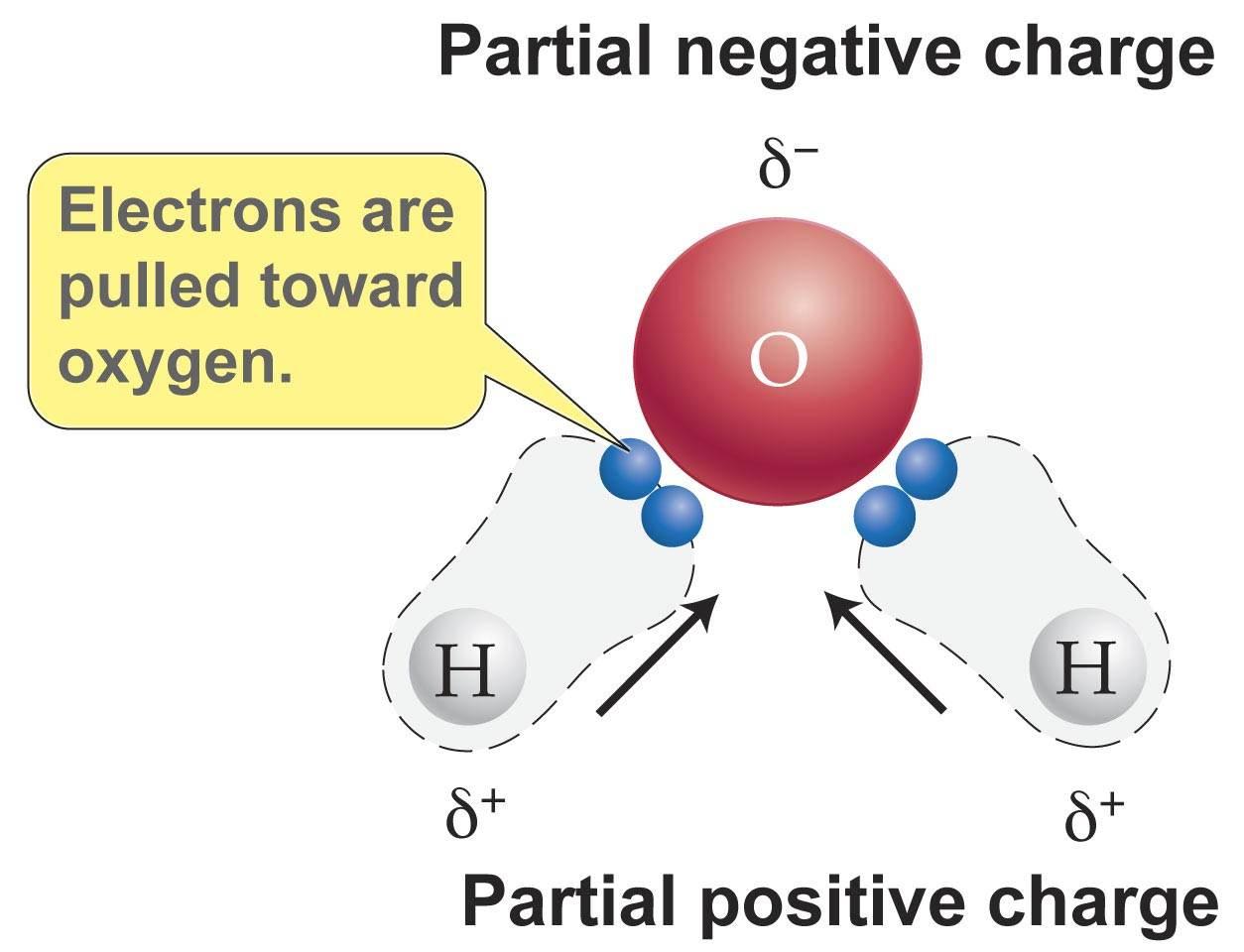
Having partial positive and partial negative charges from polar bonds arranged asymmetrically. So most of the iodine separates into it and a tiny amount stays in the water. The large difference in boiling points arises from the electronegativities of hydrogen and oxygen producing a dipole in the water molecule.
Water is a polar molecule and is attracted to other polar molecules. Water is a polar molecule. They provide cell membrane structure and resilience insulation energy storage hormones and protective barriers.
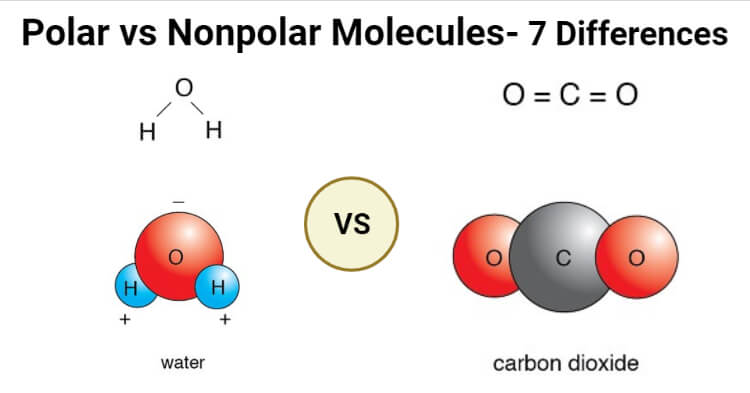
The dipoles do not cancel out resulting in a net dipole. Fats petrol oil gasoline are said to be non-polar molecules as they do not dissolve in water and nonpolar is insoluble in water. Oftentimes the bonds in the molecules of a lipid to not create charges and are nonpolar.
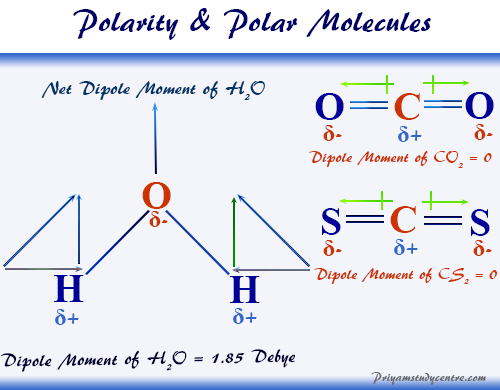
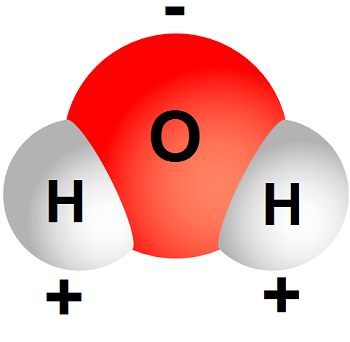
Water H 2 O is an example of a polar molecule since it has a slight positive charge on one side and a slight negative charge on the other. Some examples of molecules are. In a molecular compound such as for example water H 2 O or ethanol C 2 H 5 OH each unit of the substance the molecule consists of the number of atoms shown in the formula.
Oxygen is a highly electronegative atom when compared to hydrogen. In part c the polar covalent bonds are shown as electron. Lipids make up a group of compounds including fats oils steroids and waxes found in living organisms.
These are compound which possesses a free aldehyde or ketone group. Lipids serve many important biological roles. Its hydrogen atoms have a permanent slight positive charge.
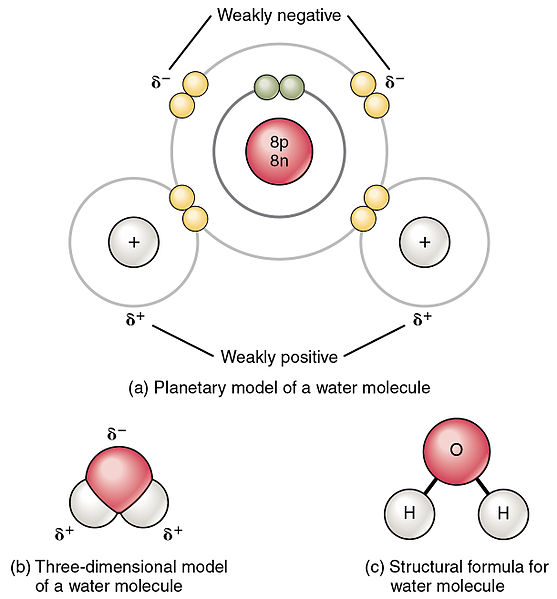
Different ways of representing the polar sharing of electrons in a water molecule. In an ionic compound such as common salt NaCl or magnesia MgO the formula tells us the correct ratio of elements present but it does not specify the unit. Water H2 O is a polar inorganic compound that is at room temperature a tasteless and odorless liquid which is nearly colorless apart from an inherent hint of blueIt is by far the most studied chemical compound and is described as the universal solvent and the solvent of life It is the most abundant substance on the surface of Earth and the only common substance to exist as a solid.
Iodine in between water and trichloromethane. A polar molecule has a net dipole as a result of the opposing charges ie. The general formula is C.
For example a polar solute such as sugar is very soluble in polar water less soluble in moderately polar methanol and practically insoluble in non-polar solvents such as benzene. Its oxygen atom has a. Water H 2 O is a polar molecule.
A polar molecule is a chemical species in which the distribution of electrons between the covalently bonded atoms is not even. H2O water N2 nitrogen O3 ozone CaO calcium oxide C6H12O6 glucose a type of. Heres an explanation of what a molecule is and is not with some examples of common molecules.
If they are highly different it can be said that the species is a highly polar molecule. Each diagram shows the unsymmetrical shape of the water molecule. Colorless crystalline solid which are soluble in water and insoluble in a non-polar solvent.
Covalent bonds in which the sharing of the electron pair is unequal with the electrons spending more time around the more nonmetallic atom are called polar covalent bonds. Though iodine is soluble in both it is non-polar in nature and hence is more soluble in trichloromethane than that in water. Solubility is the ability of a solid liquid or gaseous chemical substance.
The bonds between hydrogen and oxygen are distributed so that the hydrogen atoms are both on one side of the oxygen atom rather than evenly spaced. Lipids are molecules that dont mix well with water called hydrophobic. Another important class of molecule is the lipid class.
They also play a role in diseases. A molecule is a collection of two or more atoms that make up the smallest recognisable unit into which a pure material may be split while maintaining its makeup and chemical characteristics. Polar Molecule Definition.
The oxygen side of the molecule has a slight negative charge while the side with the hydrogen atoms has a slight positive charge. When the pull is stronger than the pull of the ionic bonds within the molecule. Water is said to be a polar molecule due to the difference in the electronegativities between the oxygen atom and the hydrogen.
Fatty acid important component of lipids in plants animals and microorganisms. Water is a polar substance which means that one end of the molecule has a different charge than the other end. Polarity is a description of how different the electrical poles of a molecule are.
Generally a fatty acid consists of a straight chain of an even number of carbon atoms with hydrogen atoms along the length of the chain and at one end of the chain and a carboxyl group COOH at the other end. Water boils at 100 C and methane boils at -1615 C.

Lesson Summary Water And Life Article Khan Academy
What Is A Polar Molecule Quora
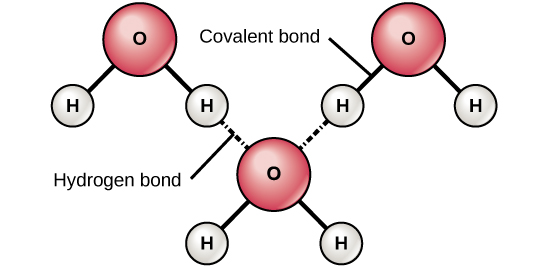
Why Life Depends On Water Biology For Non Majors I

Do Polar Molecules Dissolve In Water Why Or Why Not Quora
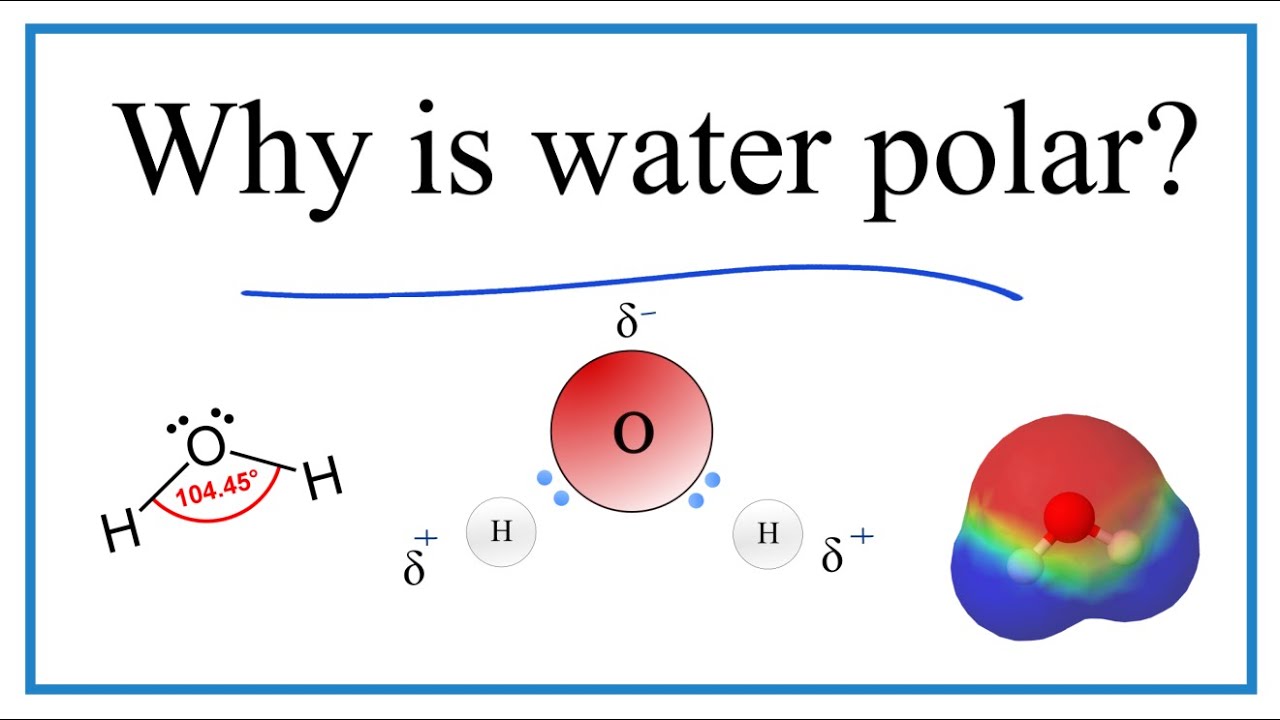
Why Is Water H2o A Polar Molecule Youtube
Polarity Of Bonds Polar Molecules Definition And Examples

Polar Covalent Bond Definition And Examples
What Is A Polar Molecule Quora
Waterproof Coating Nanoslic Coatings
Why Is Water A Polar Molecule Socratic
Chemical Polarity A Little Bit Of Physics In Your Chemistry The Institution For Science Advancement

Why Is Water Considered A Polar Molecule Quora
Polar Vs Nonpolar Molecules Definition 7 Key Differences Examples

Chemistry Ii Water And Organic Molecules
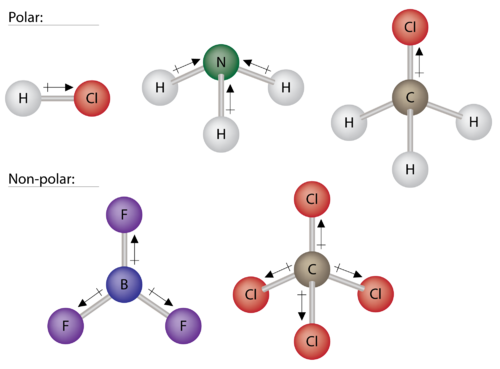
Polar Molecules Chemistry For Non Majors
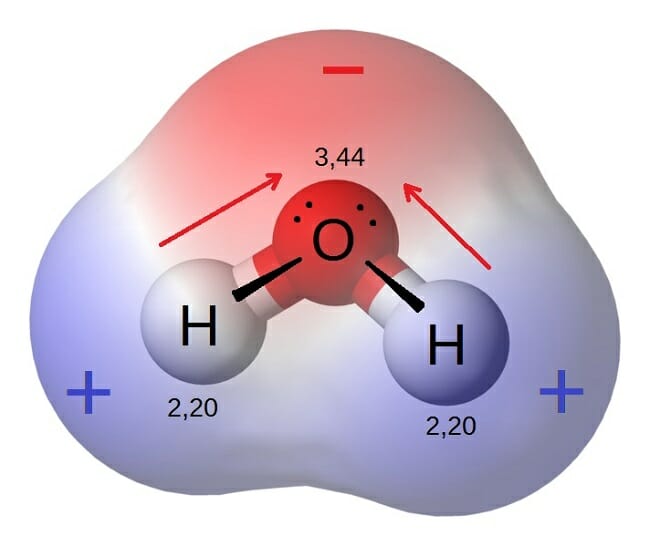
Polar Molecule Definition And Examples Biology Dictionary
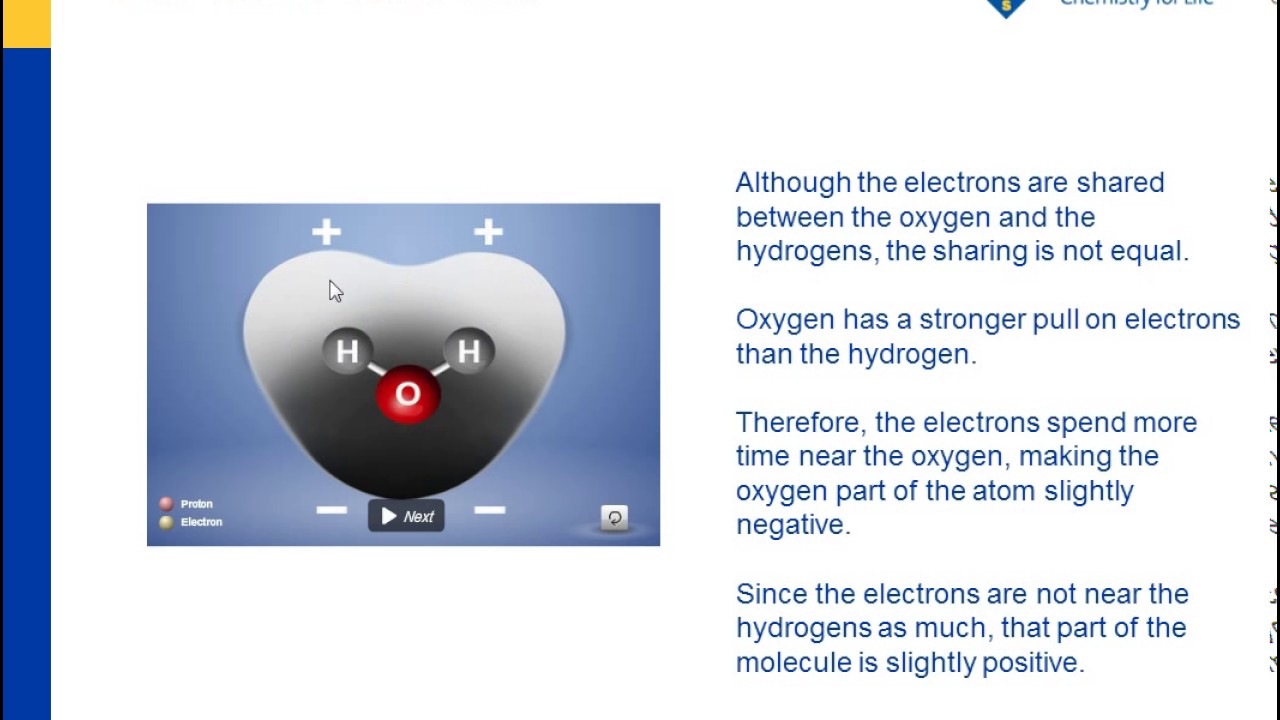
Water Is A Polar Molecule Youtube

Polar And Non Polar Molecules Tmjh 8th Grade Science
Post a Comment for "Definition Of Polar Molecule Water"