Definition Of Molecular Mass In Chemistry
The molar mass of a given substance is defined as the mass of a sample divided by the moles of that substance in the sample. Scientists call this the relative molecular mass.

1 To 20 Iupac Name Of Alkenes Names Molecular Structure Molecular
The unit in which molecular mass is.

Definition of molecular mass in chemistry. Molar volume can also be expressed in cm3 mol-1. Thus multiplying 2 to the empirical formula 2 C 3 H 2 NO 2 C 6 H 4 N 2 O 4. The mass of a single isotope of any given element the isotopic atomic mass is a value relating the mass of.
Thus the SI unit of molar volume is cubic meters per mole m3 mol-1. For most practical purposes the magnitude of molar mass is numerically the same as that of the mean mass of one molecule expressed in daltons. For example water consists of.
Finally the molecular formula is C 6 H 4 N 2 O 4. It is usually denoted by the symbol M. Molar mass is the mass of one mole of a substance given in.
Molecular mass only measures the mass of molecules and not of atoms and compounds. The sum of the atomic masses of all the atoms of each of the elements within a molecule. The mass of a molecule that is equal to the sum of the masses of all the atoms contained in the molecule.
The measurement of molecular mass is usually conducted in the case of microscopic analysis of substances. A cubic centimeter cm3 is a measure of volume one million. Relative Molecular Mass Definition Chemistry We characterize the matter as anything that has mass and occupies some space.
The amount of substance is the number of moles in the sample. Molecular mass is the mass of a single molecule given in atomic mass units amu. Molecular mass of a compound is defined as the mass of one molecule.
The molar massmolecular weight is actually the sum of the total mass in grams of the atoms present to make up a molecule per mole. In chemistry the mole is a fundamental SI unit used to measure the amount of substance. Molecular mass differs because of the isotopes.
Molecular mass is the mass of a molecule of the substance which might be different from different molecules as they may be composed of different isotopes. Let the ratio of the molar mass to empirical mass be r. Relative Atomic Mass.
The SI unit for volume is the cubic meter m3. It is likewise called atomic weight. It tends to be determined by including the mass of every atom duplicated by the number of atoms of the element present in the molecule.
In chemistry the molar volume of a substance is the volume of one mole of that substance. The units of molar mass are grams per mole abbreviated as gmol. This quantity is sometimes referred to as the chemical amount.
The weighted mean mass of an atom of an element compared to the mass of 112th a carbon-12 atom. Molecular mass is the measure of mass related to a molecule. In other words it is the mass per mole of a substance.
I show you how to work this out and then ex. A substance is something that has mass and occupies space. Since matter is characterized as whatever has mass and occupies room it ought not to be astonishing to discover that atoms and atoms have mass.
The molar mass of a substance is the mass of 1 mole of that substance in multiples of the gram. Definition of molecular mass. In this video we look at how to work out the mass of molecules.
Molar mass is defined as the mass in grams of one mole of a substance. It can be computed as the substances molecular or atomic weight divided by its density.

Gram Formula Mass In Urdu Hindi Lecture For 9th Class Lecture Formula Molar Mass

Percent Composition Easy Science Easy Science Composition Chemistry

Difference Between Formula Mass And Molecular Mass Definition Calculations With Examples Chemistry Lessons Teaching Chemistry Molecular Mass

What Is A Molecule Definition And Examples Chemical Bond Molecules Hydrogen Bond

Di Ethyl Ether Formula Diethyl Ether Ethyl Ether Molar Mass

Difference Between Formula Mass And Molecular Mass Definition Calculations With Examples Chemistry Basics Compounds And Mixtures Electron Configuration

What Is Molecular Mass Cartoon Molecular Mass Teaching Chemistry Chemistry Education

Is Methane Gas Harmful To Humans Why Methane Is A Good Fuel Methane Chemistry Education Teaching Chemistry

Average Atomic Mass Easy Science 10th Grade Science Easy Science Molar Mass

Difference Between Formula Mass And Molecular Mass Definition Calculations With Examples Chemistry Lessons Teaching Chemistry Molecular Mass

Difference Between Atom And Molecule Definition Structure Bonding Properties Chemistry Basics Science Notes Chemistry Classroom

Difference Between Empirical And Molecular Formula Chemistry Basics Chemistry Study Guide Chemistry Education

Difference Between Valence Bond Theory And Molecular Orbital Theory Definition Theory Examples In 2021 Compounds And Mixtures Chemistry Basics Pi Bond

Types Of Molecules In Urdu Hindi Lecture For 9th Class Molecules Lecture Chemistry

Atomic Mass Unit Easy Science Atomic Mass Unit 10th Grade Science Chemical Changes

Mole Concept Mole Concept Chemistry Classroom Molar Mass

Specific Volume Definition And Examples In 2021 Gas Constant Molar Mass Definitions

Toluene Formula And Structure Toluene Formula Molar Mass
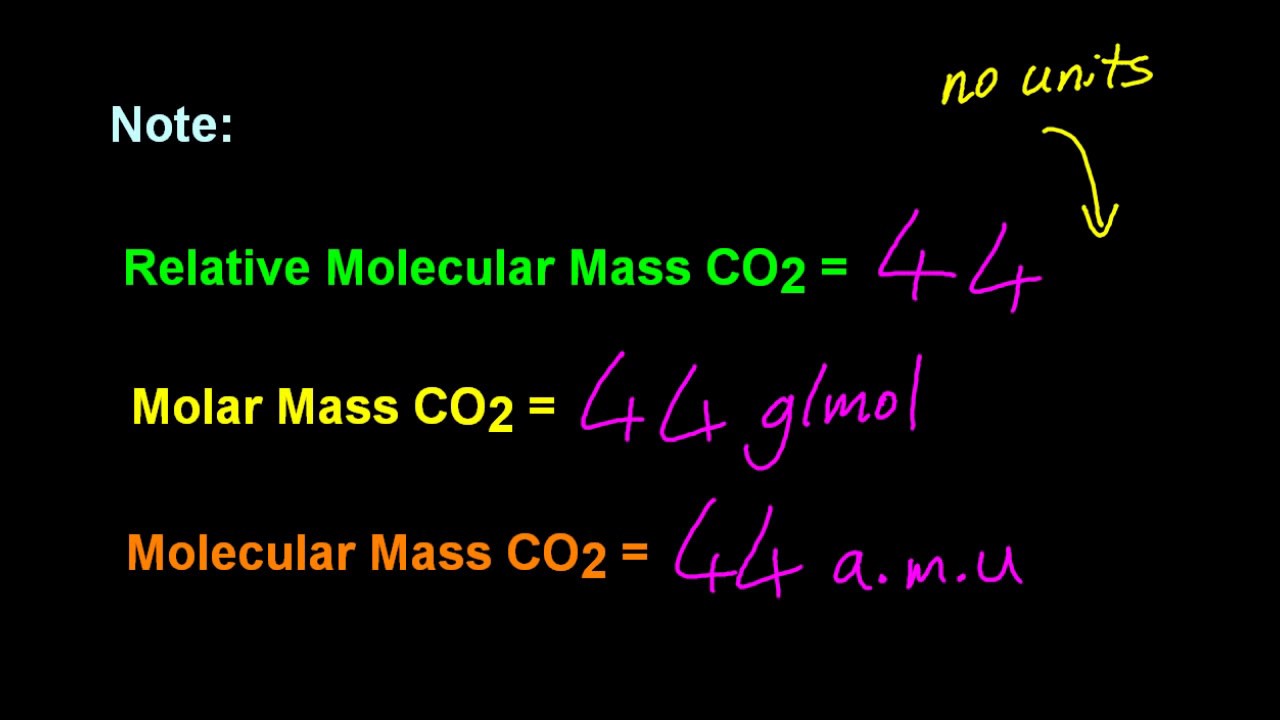
Post a Comment for "Definition Of Molecular Mass In Chemistry"