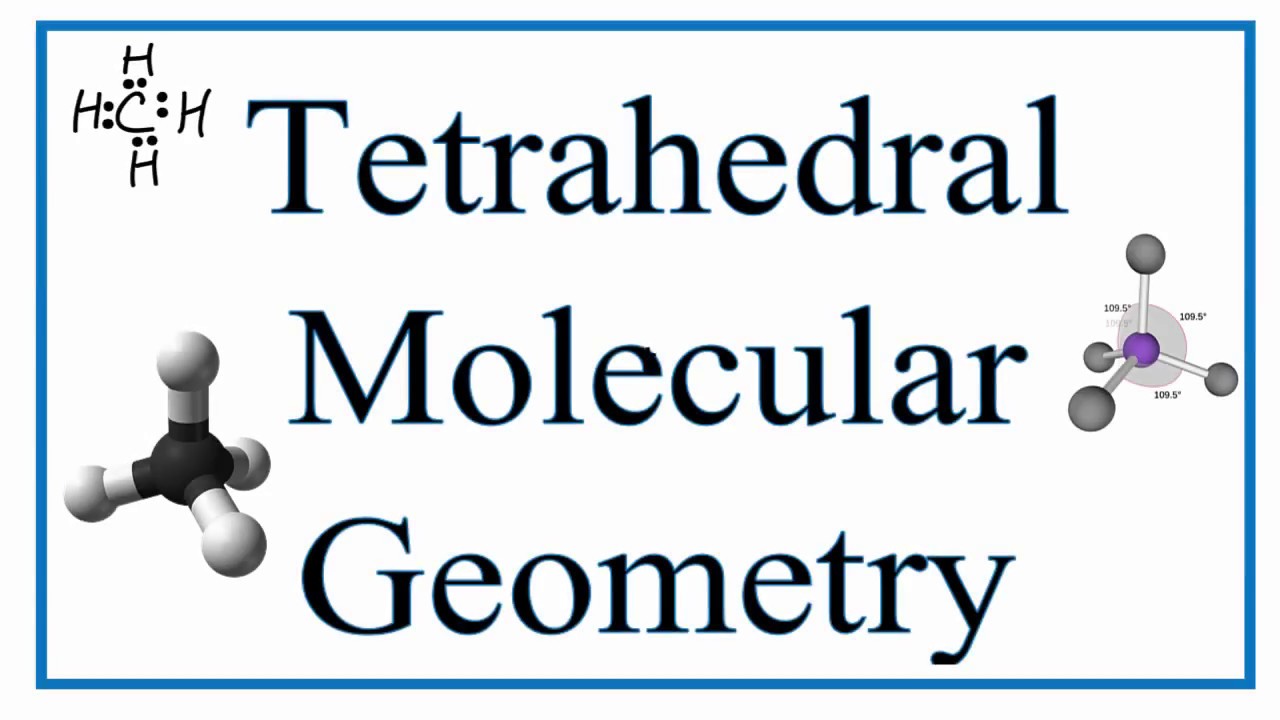
Definition Of Tetrahedral Molecule In Chemistry
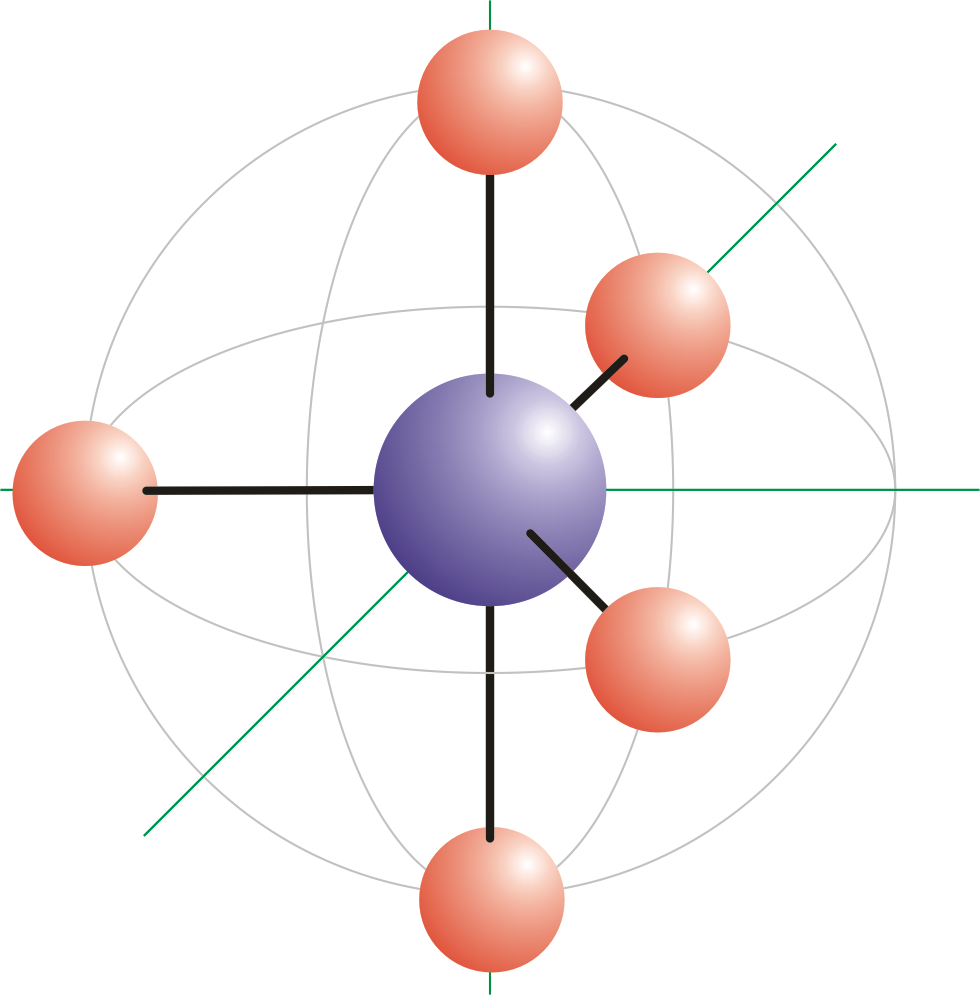
All the four hydrogen atoms are arranged in a manner such that the four hydrogen atoms form corners of a regular tetrahedron. The atoms bonded to the central atom lie at the corners of a tetrahedron with 1095 angles between them.

Linear Molecular Geometry Molecular Geometry Chemistry Projects Teaching Biology
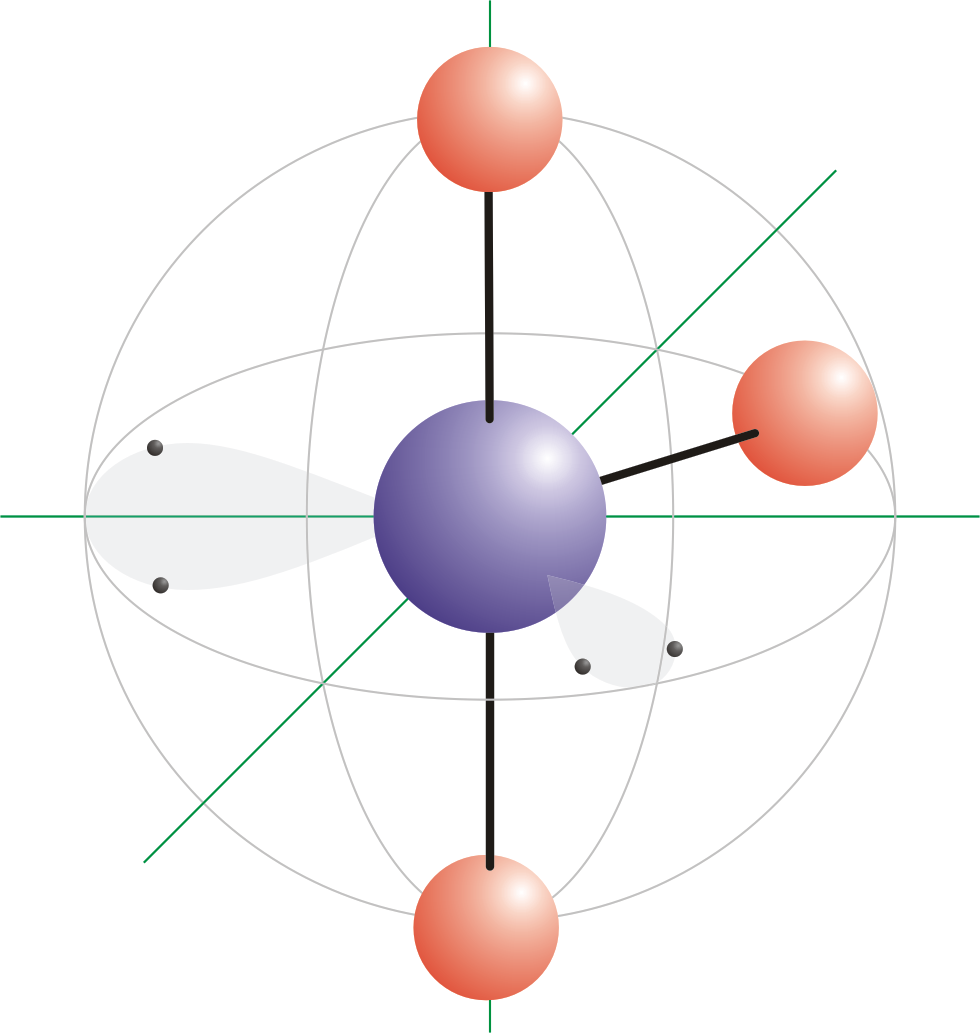
The bond angles in ammonia and in water are less than 1095 because of the stronger repulsion by their lone pairs of electrons.
Definition of tetrahedral molecule in chemistry. The concepts of steroisomerism and chirality command great deal of importance in modern organic chemistry as these ideas helps to understand the physical and theoretical reasons behind the formation and structures of numerous organic molecules the main reason behind the energy embedded in. For example the XeF 2 molecule has a steric number of five and a trigonal bipyramidal geometry. Tetra- signifies four and -hedral relates to a face of a solid.
A tetrahedral shape is a four-faced solid shape. The term tetrahedral molecular shape does not exist in the database. The bond angles are cos1 1094712206 1095 when all four substituents are the same as in methane as well as its heavier analogues.
120 three electron pairs around central atom can have molecular geometry of bent tetrahedral 1095 four electron pairs around central atom can have molecular geometry of. The tetrahedron is one kind of pyramid which is a polyhedron with a flat polygon base and triangular faces connecting the base to a common point. From the definition of Lewis acids bases in chemistry BCl 3 molecule affinity to accept electron pair acts as a Lewis acid by forming a coordinate covalent bond with other molecules or Lewis bases.
The bond angles are 10947. Based on the valence theory a covalent bond is formed between two atoms in a molecule when there is an overlapping of half-filled atomic orbitals containing unpaired electrons. These properties include fuel heat an oxidizing agent usually oxygen found in ambient air and an uninhibited chemical reaction.
The HCH bond angle in methane is the tetrahedral angle 1095. There are three possible stereoisomers. Displaying results of the search for tetrahedralmolecularshape.
Methane and other perfectly symmetrical tetrahedral molecules belong to point group Td but most tetrahedral. This shape occurs when one central atoms has four bonds. An example of a molecule with a tetrahedral shape is methane CH 4.
For example there are two different molecules with the name 2-bromobutane because there are two different ways to bond a set of four atoms or groups to a tetrahedral atom. The steric number of tetrahedral molecules is four no lone pairs. Tetrahedral molecular structure is seen in several molecules the most common of which is methane CH4.
This angle is obtained when all four pairs of outer electrons repel each other equally. Tetrahedral literally means having four faces. Physical quantities measuring units classes of compounds and materials important theories and laws.
The methane molecule is the simplest hydrocarbon of alkane or paraffin molecule having molecular formula CH 4. In accordance with the VSEPR theory the bond angles between the electron bonds are 1095 o. This stereochemical property of tetrahedral C is present in all molecules but only leads to.
Tetrahedral Geometry Molecules of methane CH4 ammonia NH3 and water H2O all have four electron groups around their central atom so they all have a tetrahedral shape and bond angles of about 1095. In a tetrahedral molecular geometry a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. Two enantiomers of a tetrahedral complex.
The tetrahedron is the three-dimensional case of the more general concept of a Euclidean simplex and may thus also be called a 3-simplex. The fire tetrahedron is a geometric representation of the four properties that must be necessary for a fire to occur within a given situation. Molecules with an tetrahedral electron pair geometries have sp3 hybridization at the central atom.
The database contains chosen terms and concepts important in chemistry and in chemistry-related fields of science eg. This shape is found when there are four bonds all on one central atom with no lone electron pairs. One in which the F atoms occupy axial sites resulting in linear molecule one in which the F atoms occupy one.
In the case of a tetrahedron the base is a triangle any of the four faces can be considered the base so a tetrahedron. Some chemical names that we have learned to inadequately describe a unique molecule. Tetrahedral molecules array four atoms around a central atom every atom oriented 1095 from the others.
Tetrahedral is a molecular shape that results when there are four bonds and no lone pairs around the central atom in the molecule. For some molecules in the Table we note that there is more than one possible shape that would satisfy the VSEPR rules. Of or having the form of a tetrahedron Meaning pronunciation translations and examples.

Shapes Of Molecules Vsepr Theory Chemistry Chemical Bonding Vsepr Theory Chemistry Chemical Bond

Trigonal Pyramid Molecular Geometry Molecular Geometry Molecular Shapes Chemistry Projects

Tetrahedral Molecular Shape Chemistry Dictionary Glossary

Molecular Geometry Chemistry Molecular Geometry Chemistry Lessons Chemistry Classroom

Tetrahedral Molecular Structure Of The Methane Download Scientific Diagram

Molecular Geometry Chemistry Worksheets Covalent Bonding Worksheet

Valence Shell Electron Pair Repulsion Theory Vsepr Diagram For High School Chemistry Students Wh High School Chemistry Teaching Chemistry Physics High School

Molecular Geometry Vsepr For Ap Chemistry Education Com Chemistry Chemistry Lessons Molecular Geometry

Molecular Geometry Boundless Chemistry

Valence Shell Electron Pair Repulsion Vsepr Chemogenesis Molecular Geometry Study Chemistry Chemistry Classroom
Tetrahedral Molecular Shape Chemistry Dictionary Glossary

Figure 11 1 General Molecular Shapes Molecular Shapes Molecular Organic Chemistry Reactions

Co Ordinate Covalent Bond Definition In Chemistry Covalent Bonding Chemistry Lessons Chemistry
Http Www Bqua Com What Is Molecular Geometry Definition

Steric Number And Bond Angles Molecular Geometry Teaching Chemistry Human Cell Structure

Tetrahedral Molecular Geometry And Bond Angles Explained Youtube

Tetrahedral In Molecular Geometry Definition Structure Examples Video Lesson Transcript Study Com
Tetrahedral Molecular Shape Chemistry Dictionary Glossary
Post a Comment for "Definition Of Tetrahedral Molecule In Chemistry"