Definition Of Work For An Expanding Gas
Consider for example a reaction that produces a gas such as dissolving a piece of copper in concentrated nitric acid. If the gas expands against the piston it exerts a force through a distance and does work on the piston.

Science Teacher High School Science Teacher Chemistry Lessons Chemistry Classroom
Parkinsons law is the adage that work expands so as to fill the time available for its completion.

Definition of work for an expanding gas. The pressure of the. That work is equal to the area under the curve on a P-V diagram which describes that expansion. The chemical equation for this reaction is as follows.
Assuming that the only work done by the reaction is work of expansion gives an equation in which the P V terms cancel. 10 C u s 4 H N O 3 a q C u N O 3 2 a q 2 H 2 O l 2 N O 2 g. Work done by an expanding gas is called pressure-volume work or just PV work.
The work done by an expanding gas is the energy transferred to its surroundings. Calculate the work done by the expanding gas on the surroundings Wenv assuming the pressure remains constant. The extreme case of this is a Joule expansion where a gas expands into a vacuum ie.
When the gas expands against an external pressure the gas has to transfer some energy to the surroundings. The work done as a result of expansion of the gas is the work of expansion. For a gas work is the product of the pressure p and the volume V during a change of volume.
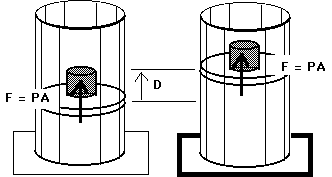
During the expansion work is done on the surroundings of magnitude where can be taken as the system pressure. The magnitude of the work done by the system is. The pressure of the surroundings times the area times the distance moved.
H q p at constant pressure. W p V We can do a quick units check to see that pressure force area times volume area length gives units of force times length which are the units of. The work is due to change of system volume by expansion or contraction of the system.
The pressure P the gas exerts on the piston is equal to the force F with which it pushes up on the piston divided by the surface area A of the piston. At constant pressure calculating work is straightforward since it is just an area of a rectangle W P Δ V. Thus the negative work decreases the overall energy of the gas.
It is sometimes applied to the growth of bureaucracy in an organization. Since the volume is increasing in this example the work done is negative the gas is doing work in the environment as it expands. The work done by a confined gas.
In thermodynamics however work has a very specific meaning. As energy is slowly added to the gas by heat the piston is pushed up a distance of 400 cm. STRATEGY The work done on the environment is the negative of the work done on the gas.
During free expansion of an ideal gas the work done is 0 be it a reversible or irreversible process. Temperature is held constant therefore the change in energy is zero U0. U q w 1 Where U represents the change in internal energy q is the heat given by the system and w is the work done on the system.
AV A Ay 0100 m2400 x 10-2 m 400 x 10-3 m3 Multiply this result by the pressure getting the work the gas does on the environment Weny Wenv PAV 101 x 105 Pa400 x 10-3 m3 404 PRACTICE IT Use the worked example above to help you solve this problem. Work Done by a Gas. The surroundings have received work.
If the system contracts in the present article it is said to do negative work on the surroundings. Therefore the change in internal energy for an isobaric process is. Compute the change in volume and multiply by the pressure.
This shows the expansion of gas at constant temperature against weight of an objects mass m on the piston. It is known that the change in internal energy of a system is given as. If the system expands in the present article it is said to do positive work on the surroundings.
So the heat absorbed by the gas equals the work done by the ideal gas on its surroundings. The amount of work done is equal to the product of the force exerted on the piston times the distance the piston is moved. Electrons moving through a potential coiling or releasing a spring and squeezing fluids hydraulic action are examples of processes which can either produce or require work.
If the piston compresses the gas as it is moved inward work is also donein this case on the gas. When a gas expands it does work on its surroundings. The work involved in the change of volume of a gas against an external pressure is of considerable interest to chemists.
As evaluated in Eq. H q p - P V P V. When the gas is compressed energy is transferred to the gas so the energy of the gas increases due to positive work.
The surroundings have given up heat numerically equal to. 453 Δ U Q P Δ V. That which results in expansion or contraction of a.
Thus the heat given off or absorbed during a chemical reaction at constant pressure is equal to the change in the enthalpy of the system. At the end of the isothermal expansion therefore. We will examine a fourth type of work in this lesson.
In effect as the gas expands it is compressing its surroundings so the work done is the force exerted on the surroundings ie.

Top 20 R Libraries For Data Science In 2018 Infographic Data Science Learning Data Science What Is Data Science
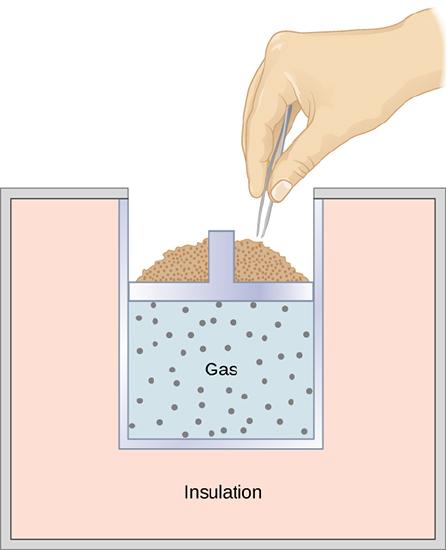
3 7 Adiabatic Processes For An Ideal Gas Physics Libretexts

Synthesis Physical Vs Chemical Properties Changes Physical Vs Chemical Properties Teaching Chemistry Chemical And Physical Changes

The Tki Package Kilmann Diagnostics Tki Assessment Critical Success Factors Teaching Writing Learning Process

Number Of Moles Mass Molar Mass Can Substitute For N In The Ideal Gas Law Giving Pv M M Rt Chemistry Education Science Biology Physics And Mathematics
Example 12 1 Work Done By An Expanding Gas Goal Apply Chegg Com

Is Methane Gas Harmful To Humans Why Methane Is A Good Fuel Methane Chemistry Education Teaching Chemistry

States Of Matter States Of Matter Solid Liquid Gas Matter

Number Of Moles Mass Molar Mass Can Substitute For N In The Ideal Gas Law Giving Pv M M Rt Chemistry Education Science Biology Physics And Mathematics
What Are Pv Diagrams Article Khan Academy
What Is The First Law Of Thermodynamics Article Khan Academy

How Lcd Projectors Work Lcd Lcd Projector Projector
What Are Pv Diagrams Article Khan Academy

6 Main Types Of Chemical Reactions Google Search Chemistry Classroom Chemistry Lessons Chemical Reactions
The First Law Of Thermodynamics




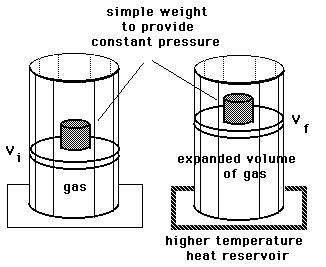

Post a Comment for "Definition Of Work For An Expanding Gas"